1- (Trifluoromethyl) -1,2-benziodoxol-3 (1 H ) -one
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1- (Trifluoromethyl) -1,2-benziodoxol-3 (1H) -one | ||||||||||||||||||
| other names |
Togni reagent II |
||||||||||||||||||
| Molecular formula | C 8 H 4 F 3 IO 2 | ||||||||||||||||||
| Brief description |
colorless, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 316.02 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
122.4–123.4 ° C (with decomposition) |
||||||||||||||||||
| solubility |
soluble in methylene chloride, chloroform, acetonitrile, methanol, ethanol, acetone |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
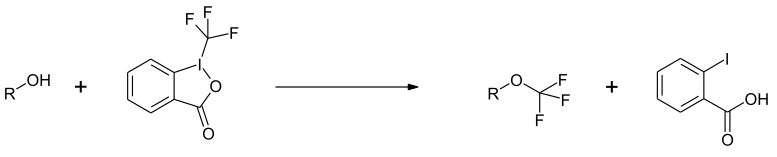
1- (Trifluoromethyl) -1,2-benziodoxol-3 (1 H ) -one (Togni reagent II) is a compound used in organic synthesis for direct electrophilic trifluoromethylation .
history
The first description of the production, properties and reactivity of the compound was made in 2006 by Antonio Togni and his colleagues from ETH Zurich . The article also provides information on 1,3-dihydro-3,3-dimethyl-1- (trifluoromethyl) -1,2-benziodoxol (Togni's reagent I).
Extraction and presentation
The synthesis takes place in a three-step process. In the first step 2-iodobenzoic acid in the presence of sodium periodate an oxidation and cyclization to give the mixed anhydride 1-hydroxy-1,2-benziodoxol-3 (1 H implemented) -one. By acylation with acetic anhydride and subsequent substitution with trimethyl (trifluoromethyl) silane , the target compound is produced.
A more recent one-pot synthesis also starts from 2-iodobenzoic acid. Trichloroisocyanuric acid is used as the oxidizing agent instead of sodium periodate .
properties
Physical Properties
The compound crystallizes in a monoclinic crystal structure. The space group is P2 1 / n with four molecules in the unit cell . A density of 2.365 g · cm −3 was derived from the crystallographic data .
Chemical properties
The pure compound is thermally stable for months at room temperature. Violent decomposition occurs above the melting point, releasing gaseous trifluoroiodomethane . The decomposition is highly exothermic. In a DSC measurement , a heat of decomposition of 502 J g −1 was determined from 149 ° C. Small amounts of trifluoromethyl-2-iodobenzoates and 2-iodobenzoyl fluoride were observed as decomposition products during recrystallization from acetonitrile . The tests regarding explosive properties are positive for the steel sleeve test and the impact sensitivity , negative for the friction sensitivity. It is therefore an explosive substance. The compound reacts violently with strong bases and acids, as well as reducing agents. Its polymerization is initiated in the solvent tetrahydrofuran .
use
The compound is used to introduce the CF 3 group into organic compounds. In the case of phenolates, there is a substitution in the ortho position. With an excess, a twofold substitution can be achieved.
The corresponding trifluoromethyl ethers are obtained with alcohols .
The CF 3 function can be added to terminal olefins with copper catalysis .
Individual evidence
- ↑ a b c d e f g h Kyrill Stanek, Raffael Koller, Iris Kieltsch, Patrick Eisenberger, Antonio Togni: 1- (Trifluoromethyl) -1,2-benziodoxol-3 (1H) -one . In: Encyclopedia of Reagents for Organic Synthesis . John Wiley & Sons, Ltd, 2001, ISBN 978-0-470-84289-8 , doi : 10.1002 / 047084289X.rn01121 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ I. Kieltsch, P. Eisenberger, K. Stanek, A. Togni: Recent Advances in Electrophilic CF 3 -Transfer Using hypervalent Iodine (III) Reagents. In: Chimia . 62, 2008, pp. 260-263, doi: 10.2533 / chimia.2008.260 .
- ↑ a b c Patrick Eisenberger, Sebastian Gischig, Antonio Togni: Novel 10-I-3 Hypervalent Iodine-Based Compounds for Electrophilic Trifluoromethylation . In: Chemistry - A European Journal . tape 12 , no. 9 , 2006, p. 2579-2586 , doi : 10.1002 / chem.200501052 .
- ↑ Václav Matoušek, Ewa Pietrasiak, Rino Schwenk, Antonio Togni: One-Pot Synthesis of Hypervalent Iodine Reagents for Electrophilic Trifluoromethylation . In: The Journal of Organic Chemistry . tape 78 , no. 13 , 2013, p. 6763-6768 , doi : 10.1021 / jo400774u .
- ↑ a b Nikolaus Fiederling, Jan Haller, Heiko Schramm: Notification about the Explosive Properties of Togni's Reagent II and One of Its precursor . In: Organic Process Research & Development . tape 17 , no. 3 , 2013, p. 318-319 , doi : 10.1021 / op400035b .
- ↑ Devoille, A .; Haller, J .: Facing the unknown with confidence: working with unidentified hazardous properties of chemical reagents in Chemistry Today 32 (3) May / June 2014, 47-50.
- ↑ Kyrill Stanek, Raffael Koller, Antonio Togni: Reactivity of a 10-I-3 Hypervalent Iodine Trifluoromethylation Reagent With Phenols . In: The Journal of Organic Chemistry . tape 73 , no. 19 , 2008, p. 7678-7685 , doi : 10.1021 / jo8014825 .
- ↑ Raffael Koller, Kyrill Stanek, Daniel Stolz, Raphael Aardoom, Katrin Niedermann, Antonio Togni: Zinc-Mediated Formation of Trifluoromethyl Ethers from Alcohols and Hypervalent Iodine Trifluoromethylation Reagents . In: Angewandte Chemie . tape 121 , no. 24 , 2009, p. 4396-4400 , doi : 10.1002 / anie.200900974 .
- ↑ Andrew T. Parsons, Stephen L. Buchwald: Copper-Catalyzed Trifluoromethylation of Unactivated Olefins . In: Angewandte Chemie . tape 123 , no. 39 , 2011, p. 9286-9289 , doi : 10.1002 / anie.201104053 .