2,6-dibromohydroquinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,6-dibromohydroquinone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 Br 2 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 267,90 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
166-167 ° C |
||||||||||||||||||
| solubility |
soluble in ethanol and diethyl ether , sparingly soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,6-Dibromohydroquinone is a chemical compound that belongs to both the polyphenols and the halogen aromatic compounds . Together with 2,3-dibromohydroquinone and 2,5-dibromohydroquinone, it is one of the three positionally isomeric dibromohydroquinones.
presentation
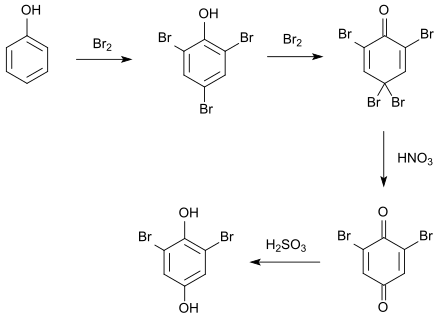
2,6-Dibromohydroquinone can be produced from phenol in a multistep reaction . First, phenol is treated with an excess of bromine , which forms 2,4,4,6-tetrabromo-2,5-cyclohexadienone (common name: tribromophenol bromine) as an intermediate via 2,4,6-tribromophenol . This is oxidized with fuming nitric acid, thereby forming 2,6-dibromoquinone, which can be reduced to 2,6-dibromohydroquinone with sulphurous acid .
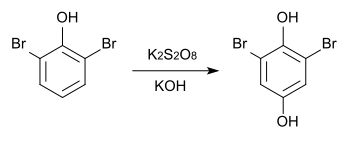
2,6-dibromohydroquinone can also be produced from 2,6-dibromophenol by Elbs oxidation .
Derivatives
In contrast to 2,5-dibromohydroquinone, the nitration of 2,6-dibromohydroquinone with nitric acid proceeds smoothly, 2,6-dibromo-3,5-dinitrohydroquinone is formed.
The reaction with dimethyl sulfate produces dimethyl ether, which has a melting point of 56 ° C and is registered under CAS number 74076-59-8.
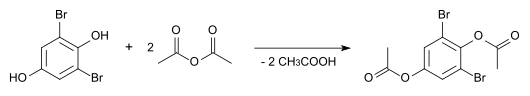
The diacetate, which can be prepared by ester formation with acetic anhydride , has a melting point of 116.5 ° C.
The reaction with chloroacetonitrile produces 2,6-dibromo-4-hydroxyphenoxyacetonitrile with a melting point of 166-167 ° C.
Occurrence in nature
2,6-dibromohydroquinone is an intermediate in the biodegradation of the herbicide bromoxynil .
Individual evidence
- ^ A b c J. Buckingham: Dictionary of Organic Compounds. CRC Press, ISBN 978-0-412-54090-5 , p. 1914 ( limited preview in Google book search).
- ↑ DA Hystazarin: "Dictionary of Organic Compounds", Volume 2. Oxford University Press, 1953. Full text
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b M. Kohn, LW Guttmann: "On the knowledge of the bromine substitution products of hydroquinone. VII. Communication on bromophenols" in monthly magazine for chemistry 1924 , 45 (10), pp. 573-588. doi : 10.1007 / BF01524599 .
- ↑ United States Patent 3860633 : 3,5-Dihalo-4-carobxyalkoxy phenols and esters thereof .
- ↑ E. Topp, LY Xun, CS Orser: Biodegradation of the herbicide bromoxynil (3,5-dibromo-4-hydroxybenzonitrile) by purified pentachlorophenol hydroxylase and whole cells of Flavobacterium sp. strain ATCC 39723 is accompanied by cyanogenesis. In: Appl Environ Microbiol. 1992, 58 (2), pp. 502-506. PMC 195275 (free full text)