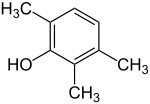
2,3,6-trimethylphenol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,3,6-trimethylphenol | ||||||||||||||||||
| other names |
3-hydroxypseudocumen |
||||||||||||||||||
| Molecular formula | C 9 H 12 O | ||||||||||||||||||
| Brief description |
White to yellowish solid smelling of phenol |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 136.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.1 g cm −3 |
||||||||||||||||||
| Melting point |
60-62 ° C |
||||||||||||||||||
| boiling point |
226 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,3,6-Trimethylphenol is a chemical compound from the group of phenols .
Occurrence
2,3,6-Trimethylphenol has been detected in coffee .
Extraction and presentation
2,3,6-Trimethylphenol can be obtained by gas-phase methylation of m- cresol with methanol at 300-460 ° C under normal pressure over orthoselective catalysts or by methylation of 2,6-xylenol on γ-aluminum oxide .
properties
2,3,6-Trimethylphenol is a flammable, hardly flammable, crystalline, white to yellowish solid with a phenol smell, which is sparingly soluble in water.
use
2,3,6-Trimethylphenol is used as an intermediate for synthetic vitamin E and for the production of 2,3,5-trimethylhydroquinone . It is also used as an intermediate for antioxidants and plastics and as a comonomer for the modification of polyphenylene oxide resins . It is also used as a flavoring agent.
Individual evidence
- ↑ a b c d e f g h Entry on 2,3,6-trimethylphenol in the GESTIS substance database of the IFA , accessed on January 30, 2019(JavaScript required) .
- ↑ a b c data sheet 2,3,6-trimethylphenol, 95% from AlfaAesar, accessed on January 30, 2019 ( PDF )(JavaScript required) .
- ↑ a b c George A. Burdock: Fenaroli's Handbook of Flavor Ingredients . CRC Press, 2016, ISBN 978-1-4200-9086-4 , pp. 1954 ( limited preview in Google Book Search).
- ↑ Entry on 2,3,6-trimethylphenol in the Hazardous Substances Data Bank , accessed January 30, 2019.
- ↑ Google Patents: CA2147913A1 - Preparation of 2,3,6-trimethylphenol - Google Patents , accessed January 30, 2019.
- ↑ Document DE3406536C2: Process for the production of 2,3,6-trimethylphenol as well as cresols and dimethylphenols, which are free from m -methyl groups, from mixtures containing meta and para cresol - document DE3406536C2 , accessed on January 30, 2019.
