2-chlorobenzylidenemalonic acid dinitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-chlorobenzylidenemalonic acid dinitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 5 ClN 2 | |||||||||||||||
| Brief description |
white crystals with a peppery odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 188.62 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.04 g cm −3 |
|||||||||||||||
| Melting point |
96 ° C |
|||||||||||||||
| boiling point |
312 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2-Chlorbenzylidenmalonsäuredinitril (after its discoverer BB C Orson and RW S also toughton (1928) CS is called) a colorless crystalline compound (also known as irritant tears tear gas takes place) use.
properties
2-Chlorobenzylidenemalonic acid dinitrile is a white crystalline, toxic, flammable solid with a pepper-like odor. If dust from the compound is whirled up, explosive mixtures with air are formed. In the event of fire or excessive heating, it decomposes into nitrous gases, hydrogen chloride and carbon oxides. 2-Chlorbenzylidenmalonsäuredinitril is poorly soluble in water and heavier than this. It slowly sinks down in water and forms toxic and strongly irritating mixtures with water, which do not lose their effect even when diluted, whereby the compound hydrolyses only slowly. It is soluble in many organic solvents, but especially in acetone , methylene chloride , dioxane , ethyl acetate and benzene .
use
As an aerosol - dissolved in dichloromethane or acetone - it is used as a police agent in riots. However, an international treaty on chemical weapons prohibits their use in war zones. The substance marketed as CS gas is used, among other things, in alarm guns and compressed air weapons , but also in small spray cans for self-defense. In Germany, in contrast to the other provisions of the Weapons Act, young people aged 14 and over are allowed to use these irritant spray devices. Gas pistols can be purchased without a permit from the age of 18, but a small gun license is required to use them in public .
For defense against dogs etc., higher CS concentrations are required than for defense against humans. In the meantime, however, the more effective so-called pepper sprays are mostly used for this purpose . The main active ingredient in these is oleoresin capsicum , or OC for short, which is obtained from chilli peppers. OC is even used in bear sprays in North America, and unlike CS, its effects are not affected by alcohol or other drug use.
Effects
2-chlorobenzylidenemalonic acid dinitrile acts directly on the neuronal pain center. The reactions to the substance vary from person to person and can also be influenced by the consumption of alcohol or other drugs . If the pain center is blocked by alcohol or other drugs, the pain sensation may be less. Around 50% of people do not react to low concentrations (0.004–0.023 mg · m −3 ), while others immediately tear and cough as a result of the irritation, and more rarely redden and itchy skin. From around 4 mg m −3 , the odor is perceived as a nuisance , with irritation of the eyes and respiratory tract occurring almost immediately. Inhaled in large quantities, CS can lead to pulmonary edema and in individual cases to death. The WHO estimates the lethal dose for humans at 25–150 g · min −1 · m −3 . In vitro , 2-chlorobenzylidenemalonic acid dinitrile showed mutagenic properties, which, however , were not reproducible in vivo .
toxicity
Although described as a non-lethal weapon for controlling crowds (e.g. during demonstrations), there are now studies that cast reasonable doubts on this assessment. CS can cause severe lung damage and severely affect the heart and liver.
On September 28, 2000, Uwe Heinrich published a study commissioned by the US Office of Special Counsel under John C. Danforth to investigate the FBI's use of CS gas in the Davidian "Mount Carmel connection" . He concluded that the lethality of CS is mainly determined by two factors: first, whether gas masks were used, and second, whether inmates were trapped in a room. He concludes that if gas masks have not been used and inmates are trapped, then "[...] it is entirely possible that this type of CS exposure could contribute significantly or even lead to death."
When CS is metabolized, cyanides can be detected in human tissue . According to the United States Army Center for Health Promotion and Preventive Medicine, CS emits very toxic fumes when heated until it decomposes. In certain concentrations, CS gas is an immediate danger to life and health. People who have been exposed to CS gas are advised to seek medical attention immediately.
Manufacturing
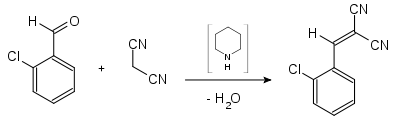
2-chlorobenzylidenemalonic acid dinitrile can be synthesized from 2-chlorobenzaldehyde and malonic acid dinitrile by Knoevenagel condensation .
See also
Web links
Individual evidence
- ↑ a b Entry on (2-chlorobenzylidene) malononitrile. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b c Entry on ((2-chlorophenyl) methylene) malonitrile in the GESTIS substance database of the IFA , accessed on October 20, 2007(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-96.
- ↑ a b L. H. Keith, DB Walters: National Toxicology Program's Chemical Solubility Compendium, National Toxicology Program (US), CRC Press, 1992, ISBN 978-0-87371-653-6 , p. 88.
- ↑ a b Data sheet 2-Chlorobenzylidenemalononitrile, 96% from Sigma-Aldrich , accessed on October 31, 2016 ( PDF ).
- ↑ Entry on 2-chlorobenzylidenemalonic acid dinitrile in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Ben B. Corson, Roger W. Stoughton: REACTIONS OF ALPHA, BETA-UNSATURATED DINITRILES. In: Journal of the American Chemical Society. 50, 1928, pp. 2825-2837, doi: 10.1021 / ja01397a037 .
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 74–75, ISBN 978-3-8348-1245-2 .
- ^ Günter Hommel: Handbook of dangerous goods. Volume 6 Springer Berlin Heidelberg, 2012, ISBN 978-3-642-25051-4 , p. 2294.
- ↑ a b c d J. P. Robinson: Public health response to biological and chemical weapons: WHO guidance. 2nd edition, WHO , 2004, ISBN 978-92-4-154615-7 , pp. 196-200.
- ^ Süddeutsche Zeitung : Blackwater used CS gas ( Memento from May 12, 2007 in the Internet Archive ), message from January 10, 2008.
- ↑ What is the difference between pepper spray and CS gas? January 18, 2017. Retrieved August 16, 2017 .
- ^ A b Howard Hu, Robert Cook-Deegan, Asfandiar Shukri: The Use of Chemical Weapons: Conducting an Investigation Using Survey Epidemiology , in: Journal of the American Medical Association , 1989 , 262 (5), pp. 640-643; PMID 2746816 .
- ^ U. Heinrich: Possible lethal effects of CS tear gas on Branch Davidians during the FBI raid on the Mount Carmel compound near Waco, Texas. (PDF; 4.1 MB) September 2000, accessed October 1, 2010.
- ↑ Kenneth E. Williams: Detailed Facts About Tear Agent O-Chlorobenzylidene Malononitrile (CS). US Army Center for Health Promotion and Preventive Medicine. Accessed on September 23, 2007 (PDF; 25 kB).