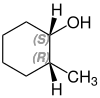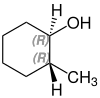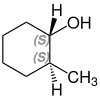2-methylcyclohexanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
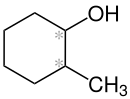
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry (* = stereocenter) |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-methylcyclohexanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 14 O | ||||||||||||||||||
| Brief description |
colorless liquid with a camphor-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 114.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Vapor pressure |
3.5 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.462 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2-Methylcyclohexanol is a chemical compound from the group of cyclohexanol derivatives , so it is one of the alcohols .
Stereoisomerism
2-Methylcyclohexanol has two stereocenters, so there are four stereoisomers :
- (1 S , 2 R ) -2-methylcyclohexanol
- (1 R , 2 S ) -2-methylcyclohexanol
- (1 R , 2 R ) -2-methylcyclohexanol
- (1 S , 2 S ) -2-methylcyclohexanol
A mixture of (1 S , 2 R ) -2-methylcyclohexanol and (1 R , 2 S ) -2-methylcyclohexanol is called cis -2-methylcyclohexanol. trans -2-methylcyclohexanol is a mixture of (1 R , 2 R ) -2-methylcyclohexanol and (1 S , 2 S ) -2-methylcyclohexanol.
Extraction and presentation
trans -2-methylcyclohexanol can be obtained by hydroboration of methylcyclohexene . A mixture of 75% cis -2-methylcyclohexanol and 25% trans -2-methylcyclohexanol can be obtained by reducing 2-methylcyclohexanone with lithium triethylborohydride .
properties
The compound is a colorless liquid with a camphor-like odor that is sparingly soluble in water.
use
2-Methylcyclohexanol is used to study the effect of organic solvents on epoxide hydrolase. It is also used in the production of acetic acid (2-methyl-cyclohexyl ester ) by reaction with acetic anhydride .
safety instructions
The vapors of 2-methylcyclohexanol can form an explosive mixture with air ( flash point 58 ° C, ignition temperature 295 ° C).
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q Entry on 2-methylcyclohexanol in the GESTIS substance database of the IFA , accessed on January 12, 2019(JavaScript required) .
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 378 ( limited preview in Google Book search).
- ↑ a b Data sheet 2-Methylcyclohexanol, mixture of cis and trans, 99% from Sigma-Aldrich , accessed on January 12, 2019 ( PDF ).
- ↑ Entry on 2-methylcyclohexanol, mixed isomers in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 12, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ BAuA: Explanation on methylcyclohexanol in TRGS 900 , June 2008, accessed on January 12, 2019.
- ↑ ScienceDirect Topics: Hydroboration - an overview | ScienceDirect Topics , accessed January 12, 2019.
- ^ Marye Anne Fox, James K. Whitesell: Organic Chemistry . Jones & Bartlett Learning, 2004, ISBN 978-0-7637-2197-8 , pp. 498 ( limited preview in Google Book search).
- ^ Herbert C. Brown, SC Kim, S. Krishnamurthy: Selective reductions. 26. Lithium triethylborohydride as an exceptionally powerful and selective reducing agent in organic synthesis. Exploration of the reactions with selected organic compounds containing representative functional groups. In: The Journal of Organic Chemistry. 45, 1980, p. 1, doi : 10.1021 / jo01289a001 .
- ↑ Data sheet 2-methylcyclohexanol, cis + trans, 97% from AlfaAesar, accessed on January 12, 2019 ( PDF )(JavaScript required) .