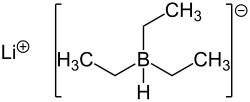
Lithium triethyl borohydride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lithium triethyl borohydride | ||||||||||||||||||
| other names |
Lithium triethylhydridoborate |
||||||||||||||||||
| Molecular formula | C 6 H 16 BLi | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 105.94 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.892 g cm −3 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lithium triethylborohydride is a chemical compound from the group of organoboron compounds .
Extraction and presentation
Lithium triethyl borohydride can be obtained by reacting lithium hydride with triethyl borane in diethyl ether at 0 ° C. Synthesis by reaction of triethylborane with tert- butyllithium or lithium aluminum hydride in the presence of triethylenediamine is also possible . Other display methods are also known.
properties
Lithium triethylborohydride is a liquid that is miscible with tetrahydrofuran and benzene.
use
Lithium triethyl borohydride is used as a strong reducing agent in organic syntheses for converting carbonyl compounds into alcohols , in the production of alkynyl alcohols from the cleavage of cyclic ketovinyl triflates or in the reduction of esters and lactones to alcohols or diols . It is also used to produce 1-methylcyclohexanol and 1,4-butanediol from 1,2-epoxybutane or gamma-butyrolactone , and to produce 2-methylcyclohexanol from 2-methylcyclohexanone . It is also used for the reductive cleavage of mesylates and tosylates in synthetic organic chemistry.
Individual evidence
- ↑ a b c d e f g h data sheet Lithium triethylborohydride, 1M in THF from AlfaAesar, accessed on January 30, 2019 ( PDF )(JavaScript required) .
- ↑ Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 6 Boron Compounds . Georg Thieme Verlag, 2014, ISBN 3-13-171761-0 , p. 84 ( limited preview in Google Book search).
- ↑ Marek Zaidlewicz, Herbert C. Brown, Ameya S. Kulkarni, P. Veeraraghavan Ramachandran: Lithium Triethylborohydride . In: Encyclopedia of Reagents for Organic Synthesis. doi : 10.1002 / 047084289X.rl148.pub2
- ^ EW Abel: Organometallic Chemistry . Royal Society of Chemistry, 1973, ISBN 978-0-85186-511-9 , pp. 73 ( limited preview in Google Book search).
- ^ Herbert C. Brown, SC Kim, S. Krishnamurthy: Selective reductions. 26. Lithium triethylborohydride as an exceptionally powerful and selective reducing agent in organic synthesis. Exploration of the reactions with selected organic compounds containing representative functional groups. In: The Journal of Organic Chemistry. 45, 1980, p. 1, doi : 10.1021 / jo01289a001 .
- ^ S. Krishnamurthy, Herbert C. Brown: Selective reductions. 31. Lithium triethylborohydride as an exceptionally powerful nucleophile. A new and remarkably rapid methodology for the hydrogenolysis of alkyl halides under mild conditions. In: The Journal of Organic Chemistry. 48, 1983, p. 3085, doi : 10.1021 / jo00166a031 .


