2-propyl-1-heptanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-propyl-1-heptanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 22 O | |||||||||||||||
| Brief description |
colorless liquid with a mild odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 158.28 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.8323 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−116.6 ° C |
|||||||||||||||
| boiling point |
218.4 ° C |
|||||||||||||||
| Vapor pressure |
0.021 h Pa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4371 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Propyl-1-heptanol (short: 2-PH ) is a fully synthetic, branched primary alcohol with a mild alcohol-like odor. It is of little importance as a solvent . Most of it is chemically processed.
Isomerism
2-Propylheptan-1-ol occurs in two enantiomeric forms because it has a center of chirality on the C2 carbon . Thus, there exist (R) -2-propylheptan-1-ol and (S) -2-propylheptan-1-ol. A 1: 1 mixture of the two enantiomers is called a racemate .
Extraction and presentation
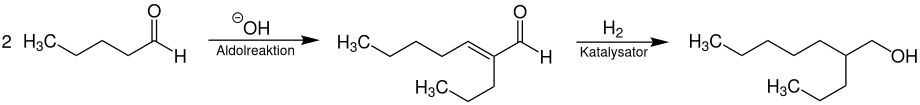
The technical production of racemic 2-propyl-1-heptanol takes place by aldol condensation of valeraldehyde , which is first converted to 2-propylhept-2-enal. After a catalytic hydrogenation , 2-propyl-1-heptanol is obtained.
properties
Physical Properties
2-Propylheptan-1-ol has a relative gas density of 5.47 (density ratio to dry air at the same temperature and pressure ) and a density of 0.8323 g / cm 3 at 20 ° C. It also has a vapor pressure of 0.021 hPa at 25 ° C. The dynamic viscosity at 20 ° C. is given as 15.3 mPas.
Chemical properties
2-Propylheptan-1-ol is a flammable, hardly inflammable liquid from the group of alcohols . It is difficult or very difficult to volatilize . 2-Propylheptan-1-ol is lighter than water and practically insoluble in it (58 mg · l −1 at 20 ° C).
use
2-Propyl-1-heptanol is used as an intermediate in the production of other chemical compounds (e.g. the plasticizer DPHP ).
safety instructions
2-Propyl-1-heptanol is a hardly flammable liquid . The substance is classified as hazardous to water , so it is harmful to aquatic organisms with long-term effects. Ingestion or contact can cause severe irritation to the eyes and skin . 2-Propyl-1-heptanol has a lower explosion limit (LEL) of 0.8% by volume and an upper explosion limit (UEL) of 5.7% by volume. The ignition temperature is 265 ° C. The substance therefore falls into temperature class T3. With a flash point of 100 ° C, 2-propyl-1-heptanol is considered flame-retardant.
See also
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2-propylheptan-1-ol in the GESTIS substance database of the IFA , accessed on January 27, 2020(JavaScript required) .
- ↑ a b Propylheptanol. In: BASF product search. BASF SE, 2014, accessed January 30, 2019 (German, English).
- ↑ Propylheptanol. In: BASF Technical Information. BASF SE, December 2014, accessed on January 30, 2019 .
- ↑ Entry on 2-propylheptan-1-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 30, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 2-n-propyl-1-heptanol, 98% from AlfaAesar, accessed on January 30, 2019 ( PDF )(JavaScript required) .
- ^ Armin Börner, Robert Franke: Hydroformylation Fundamentals, Processes, and Applications in Organic Synthesis . John Wiley & Sons, 2016, ISBN 978-3-527-33552-7 , pp. 290 ( limited preview in Google Book Search).