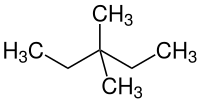
3,3-dimethylpentane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3,3-dimethylpentane | |||||||||||||||
| Molecular formula | C 7 H 16 | |||||||||||||||
| Brief description |
highly flammable colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.69 g cm −3 |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
86 ° C |
|||||||||||||||
| Vapor pressure |
191 hPa (37.7 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (5.9 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3,3-Dimethylpentane is a chemical compound from the group of aliphatic saturated hydrocarbons . It is one of the nine constitutional isomers of heptane .
Extraction and presentation
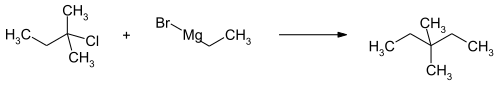
3,3-Dimethylpentane can be obtained by the isomerization of n -heptane , where the compound has to be separated from the resulting mixture of isomers. A laboratory synthesis takes place via the conversion of the Grignard compound from ethyl bromide with amyl chloride with 2-chloro-2-methylbutane .
properties
Physical Properties
3,3-Dimethylpentane is a highly flammable and colorless liquid. According to Antoine, the vapor pressure function results according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.95568, B = 1230.986 and C = −47.568 in the temperature range from 287 to 360 K. The temperature dependence of the enthalpy of vaporization can be calculated according to the equation Δ V H 0 = A · e (−βT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature) with A = 47.53 kJ / mol, β = 0.2661 and T c = 536.3 K in the temperature range between 298 K and 359 K. In the solid phase, there are two polymorphic crystal forms that show different melting points. Both forms are enantiotropic to one another. The transition temperature between the two forms is −140.45 ° C.
The most important thermodynamic properties are listed in the following table:
| property | Type | Value [unit] |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 gas Δ f H 0 liquid |
−201.5 kJ mol −1 −234.6 kJ mol −1 |
| Enthalpy of combustion | Δ c H 0 liquid | −4806.70 kJ mol −1 |
| Heat capacity | c p | 214.8 J mol −1 K −1 (25 ° C) as a liquid |
| Enthalpy of fusion | Δ f H 0 | 6.8463 kJ mol −1 polymorph I 7.6425 kJ mol −1 polymorph II |
| Entropy of fusion | Δ f S 0 | 49.34 kJ mol −1 polymorph I 55.30 kJ mol −1 polymorph II |
| Enthalpy of transformation | Δ t H 0 | 0.7937 kJ mol −1 polymorph II to polymorph I. |
| Entropy of transformation | Δ t S 0 | 5.98 kJ mol −1 polymorph II to polymorph I. |
| Enthalpy of evaporation | Δ V H 0 | 29.62 kJ mol −1 at the normal pressure boiling point 33.15 kJ mol −1 at 25 ° C |
| Critical temperature | T C | 263.25 ° C |
| Critical pressure | P C | 29.5 bar |
| Critical volume | V C | 0.414 l mol −1 |
| Critical density | ρ C | 2.42 mol·l −1 |
Safety-related parameters
3,3-Dimethylpentane forms highly flammable vapor-air mixtures. The compound has a flash point of −15 ° C. The explosion range lies between 0.9% by volume (40 g / m 3 ) as the lower explosion limit (LEL) and 6.9% by volume (285 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 320 ° C. The substance therefore falls into temperature class T2.
Historical
In 1866 and 1867 Charles Friedel and Albert Ladenburg reported on a synthesis of 3,3-dimethylpentane, which they called “carbdimethyl diethyl”, starting from 2,2-dichloropropane.
Individual evidence
- ↑ a b c d e f g h i Entry on 3,3-dimethylpentane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e f g h i j Finke, HL; Messerly, JF; Douslin, DR: Low-temperature thermal quantities for five alkyl-substituted pentanes in J. Chem. Thermodynam. 8 (1976) 965-983, doi : 10.1016 / 0021-9614 (76) 90113-0 .
- ↑ Houzvicka, J .; Blom, NJ: Patent US2004 / 59174 A1, 2004.
- ↑ Gillespie, RD: Patent US2004 / 249231 A1, 2004.
- ↑ Herbst, K .; Stern, P .; Blom, NJ; Starch-Hytoft, G .; Knudsen, KG: Patent US2007 / 123745 A1, 2007.
- ↑ marker, RE; Oakwood, TS: Hexamethylethane and Tetraalkylmethanes in J. Am. Chem. Soc. 60 (1938) 2598, doi : 10.1021 / ja01278a011 .
- ↑ Forziati, AF; Norris, WR; Rossini, FD: Vapor Pressures and Boiling Points of Sixty API-NBS Hydrocarbons in J. Res. Natl. Bur. Stand. (US) 43 (1949) 555-563, doi : 10.6028 / jres.043.050 .
- ↑ a b c Majer, V .; Svoboda, V .: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, p. 300.
- ↑ a b c Prosen, EJ; Rossini, FD: Heats of combustion and formation of the paraffin hydrocarbons at 25 ° C in J. Res. Natl.Bur. Status 1945, 263–267.
- ↑ a b c d Daubert, TE: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes in J. Chem. Eng. Data 41 (1996) 365-372, doi : 10.1021 / je9501548 .
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ^ Charles Friedel , Albert Ladenburg : About the synthesis of a hydrocarbon and its constitution . In: Friedrich Wöhler, Justus Liebig, Hermann Kopp (eds.): Justus Liebig's annals of chemistry . tape 142 , no. 3 . CF Winter'sche Verlagshandlung / Wiley-VCH, Leipzig and Heidelberg 1867, p. 310–322 , doi : 10.1002 / jlac.18671420310 ( online at the Bayerische Staatsbibliothek BSB / Munich digitization center MDZ ).