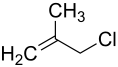
3-chloro-2-methylpropene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-chloro-2-methylpropene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 7 Cl | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 90.55 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.93 g cm −3 |
|||||||||||||||
| Melting point |
<−80 ° C |
|||||||||||||||
| boiling point |
72 ° C |
|||||||||||||||
| Vapor pressure |
138 hPa (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (0.5 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4245 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-chloro-2-methylpropene is a colorless, pungent smelling liquid. It belongs to the group of unsaturated halogenated hydrocarbons or halogen alkenes .
Extraction and presentation
It is by chlorination of 2-methyl-1-propene prepared.
properties
3-chloro-2-methylpropene is a volatile, colorless, polymerizable liquid with a pungent odor that is very sparingly soluble in water. It decomposes when heated, which can produce hydrogen chloride , chlorine and other toxic or corrosive substances.
use
3-chloro-2-methylpropene is used as an intermediate in the manufacture of insecticides .
safety instructions
The vapors of 3-chloro-2-methylpropene form an explosive mixture with air ( flash point −12 ° C, ignition temperature 540 ° C).
Web links
- 3-chloro-2-methylpropene [MAK Value Documentation, 1992]. The MAK Collection for Occupational Health and Safety. 2012, pp. 98-106 doi: 10.1002 / 3527600418.mb56347e0004
Individual evidence
- ↑ a b c d e f g h i j k l Entry on 3-chloro-2-methylpropene in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Francis A. Daniher, Peter E. Butler: addition of N, N-dichlorosulfonamides to unsaturates . In: The Journal of Organic Chemistry . tape 33 , no. December 12 , 1968, p. 4336-4340 , doi : 10.1021 / jo01276a006 .
- ↑ Entry on 3-chloro-2-methylpropene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Otto Schales: Experiments with 3-chloro-2-methyl-propene (1). In: Reports of the German Chemical Society (A and B Series). 70, 1937, pp. 116-121, doi: 10.1002 / cber.19370700125 .



