4-butyl-1,3-benzenediol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
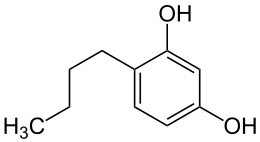
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-butyl-1,3-benzenediol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 14 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 166.22 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-butyl-1,3-benzenediol (name according to IUPAC ), also 4-butylresorcinol ( INCI ) or rucinol ( name protected by Pola Chemical Industries ), is a synthetic compound and is used as an active ingredient for skin lightening . One area of application is, for example, the treatment of hyperpigmentation . Hyperpigmentation is caused by an increased production of the body's own skin pigment melanin , for example lentigo or age spots .
The active ingredient, which was developed in Japan over ten years, is intended to specifically interrupt melanin production by inhibiting two enzymes required for melanin synthesis ( melanogenesis ) : tyrosinase and TRP-1 . First, melanin production is generally inhibited, then the production of black melanin, which is responsible for the intense coloration of the pigment spots, is specifically blocked.
The result of the double intervention in the hyperpigmentation is a complete discoloration of the pigment spots. The effect of 4-butylresorcinol on melanin production is said to be around 100 times stronger than that of hydroquinone and more than five times stronger than that of kojic acid .
In Germany, cosmetics containing 4-butylresorcinol (rucinol) have been available in the form of serum and cream since January 2005 .
Individual evidence
- ↑ Entry on 4-BUTYLRESORCINOL in the CosIng database of the EU Commission, accessed on April 27, 2020.
- ↑ a b Data sheet 4-butyl-resorcinol from Sigma-Aldrich , accessed on January 9, 2020 ( PDF ).
- ↑ Entry on 4-butyl-1,3-benzenediol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Dong-Seok Kim, So-Young Kim, Seo-Hyoung Park, Yeong-Gon Choi, Sun-Bang Kwon, Myo-Kyoung Kim, Jung-Im Na, Sang-Woong Youn, Kyoung-Chan Park: Inhibitory effects of 4 -n-butylresorcinol on tyrosinase activity and melanin synthesis . In: Biological and Pharmaceutical Bulletin . tape 28 , no. 12 , 2005, p. 2216–2219 , doi : 10.1248 / bpb.28.2216 , PMID 16327152 (free full text).
- ↑ Takayuki Katagiri, Tadashi Okubo, Midori Oyobikawa, Kiyoko Futaki, Masao Shaku, Mitsuo Kawai, Masaki Takenouchi: Inhibitory Action of 4-n-Butylresorcinol (Rucinol®) on Melanogenesis and Skin Whitening Effects . In: Journal of Society of Cosmetic Chemists of Japan . tape 35 , no. 1 , 2001, p. 42–49 , doi : 10.5107 / sccj.35.42 (free full text).
- ^ Combatting pigment spots causally, Pharmazeutische Zeitung, June 6, 2005.