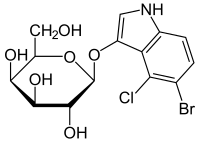
5-Bromo-4-chloro-3-indoxyl-β-D-galactopyranoside
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 5-Bromo-4-chloro-3-indoxyl-β-D-galactopyranoside | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 15 BrClNO 6 | |||||||||||||||
| Brief description |
colorless crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 408.6 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
236 ° C (decomposition) |
|||||||||||||||
| solubility |
soluble in DMSO |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
5-Bromo-4-chloro-3-indoxyl-β-D-galactopyranoside , also X-Gal , is an artificial glycoside and a chromogenic substrate for the enzyme β-galactosidase .
properties
The enzyme β-galactosidase hydrolyzes X-Gal to galactose and 5-bromo-4- chloroindoxyl (5-bromo-4-chloro-3-hydroxyindole). The 5-bromo-4-chloro-indoxyl is oxidized by the oxygen in the air to the deep blue dye 5,5'-dibromo-4,4'-dichloro- indigo .
use
In biochemistry , X-Gal is used for the qualitative determination of the activity of β-galactosidase. In contrast, the substrate o-nitrophenyl-β-D-galactopyranoside (ONPG) is used for quantitative determination .
In molecular biology , X-Gal is used for blue-white selection . In many cloning vectors is in the amino terminal coding sequence of β-galactosidase (α-fragment) a multiple cloning site into the foreign DNA, such as. B. PCR fragments can be introduced. This disrupts the β-galactosidase gene sequence and the enzyme is no longer expressed. From this it follows that the X-Gal is no longer split and therefore no blue dye is formed. The colonies formed after the transformation then do not appear blue like those with an empty vector , but white.
X-Gal is often used in conjunction with IPTG (for the induction of the lac promoter , see also lac operon ) for the screening of blue colonies. It is also used for the screening of β-galactosidase reporter gene activities in transfections of eukaryotic cells and for the detection of β-galactosidase in immunology and histochemical applications.
Other chromogenic substrates:
- 5-bromo-3-indolyl-β-D-galactopyranoside (Blue-Gal, Bluo-Gal)
- 6-chloro-3-indolyl-β-D-galactopyranoside (Y-Gal, Red-Gal, Rose-Gal, Salmon-Gal)
- 5-iodo-3-indolyl-β-D-galactopyranoside (Purple-Gal)
- 5-bromo-6-chloro-3-indolyl-β-D-galactopyranoside (Magenta-Gal)
- N -Methylindolyl-β-D-galactopyranoside (Green-Gal)
Other fluorogenic substrates:
- 4-methyl-umbelliferyl-β-D-galactopyranoside (MUG) (λ ex = 365 nm, λ em = 455 nm)
See also
Individual evidence
- ↑ a b c d e data sheet 5-Bromo-4-chloro-3-indolyl-beta-D-galactopyranoside, 98 +% from AlfaAesar, accessed on December 25, 2019 ( PDF )(JavaScript required) .
literature
- Sambrook, J .; Maniatis, T .; Russel, DW: Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; 3rd edition (2001) ISBN 0-87969-577-3 .
- Horwitz, JP et al. (1964). Substrates for Cytochemical Demonstration of Enzyme Activity. I. Some Substituted 3-Indolyl-β-D-glycopyranosides. J Med Chem. 7 (4), pp. 574-575; doi: 10.1021 / jm00334a044 .
- Edwards, MJ, and Taylor, MF (1993). Substitution of DMSO for DMF as a Solvent for X-Gal. BioTechniques, 14, p. 234.

