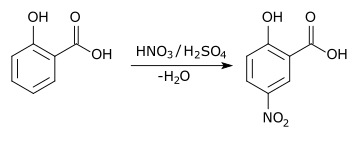
5-nitrosalicylic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 5-nitrosalicylic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 5 NO 5 | ||||||||||||||||||
| Brief description |
orange-red solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 183.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.65 g cm −3 |
||||||||||||||||||
| Melting point |
228-231 ° C |
||||||||||||||||||
| solubility |
poor in water (1.76 g l −1 at 22 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
5-Nitrosalicylic acid is an organic chemical compound that belongs to the group of phenols as well as the group of aromatic carboxylic acids . It is therefore a phenolic acid .
presentation
5-Nitrosalicylic acid can be obtained from salicylic acid by nitration in a sulfuric acid solution.
use
5-Nitrosalicylic acid is used as the starting material for the production of 5-aminosalicylic acid (mesalazine), which in turn is the starting material for numerous pharmaceutical products (world demand 300 tons / year).
Reactions
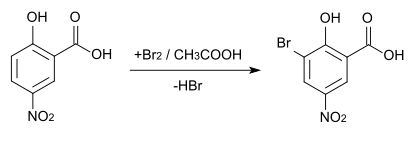
Brominating 5-nitrosalicylic acid with elemental bromine in glacial acetic acid gives 3-bromo-5-nitrosalicylic acid , the melting point of which is 222 ° C.
Via this 3-bromo-5-nitrosalicylic acid, 3-bromosalicylic acid (CAS number: 3883-95-2, melting point at 184 ° C) is also accessible by reduction and diazotization.
Web links
Individual evidence
- ↑ a b c d Data Sheet 5-nitrosalicylic (PDF) at Merck , accessed March 9, 2010 .
- ↑ a b Entry on 5-nitrosalicylic acid in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ KS Webb, V. Seneviratne: A mild oxidation of aromatic amines , in: Tetrahedron Lett. , 1995 , 36 , pp. 2377-2378; doi: 10.1016 / 0040-4039 (95) 00281-G .
- ^ G. Breviglieri, B. Giacomo, C. Sergio, A. Cinzia, E. Campanab, M. Panunzio: Reduction of 5-Nitrosalicylic acid in water to give 5-Aminosalicylic acid , in: Molecules , 2001 , 6 , M260, doi: 10.3390 / M260 .
- ↑ a b c E. Lellmann, R. Grothmann, Ueber some derivatives of salicylic acid , in: Chem. Ber. , 1884 , 17 , pp. 2724-2731.