Aconitic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
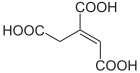
|
||||||||||||||||
| Structural formulas of cis and trans aconitic acid | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Aconitic acid | |||||||||||||||
| other names |
1,2,3-propentricarboxylic acid |
|||||||||||||||
| Molecular formula | C 6 H 6 O 6 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 174.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.56 g cm −3 ( trans ) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
very good in water (400 g l −1 at 20 ° C, trans ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Aconitic acid is the common name for 1,2,3-propentricarboxylic acid , an unsaturated organic compound with three carboxylic acid functions . Their salts are called aconites.
Isomers
The aconitic may as cis - or trans - isomer present.
| Isomers of aconitic acid | ||
| Surname | cis aconitic acid | trans -aconitic acid |
| other names | ( Z ) -aconitic acid | ( E ) -aconitic acid |
| Structural formula |
|

|
| CAS number | 585-84-2 | 4023-65-8 |
| 499-12-7 (unspec.) | ||
| EC number | 209-564-4 | 223-688-6 |
| 207-877-0 (unspec.) | ||
| ECHA info card | 100.008.697 | 100,021,536 |
| 100.007.162 (unspec.) | ||
| PubChem | 643757 | 444212 |
| 309 (unspec.) | ||
| Wikidata | Q27104226 | Q27104227 |
| Q288782 (unspec.) | ||
Occurrence
Both isomers of aconitic acid occur in nature.
The cis aconitic acid is an intermediate product in the conversion of citrate to isocitrate by the aconitase in the citric acid cycle and glyoxylate cycle .
It occurs in free form in the blue monkshood flower ( Aconitum napellus ), but also in other plants such as the common yarrow ( Achillea millefolium ) and the Christmas rose .
presentation
Aconitic can be achieved by dehydration of citric acid produced under the influence of concentrated sulfuric acid:
It was first produced in 1875 from citric acid thermally at 170 ° C.
properties
The cis -isomer very easily forms an anhydride , cis -aconitic anhydride with a melting point of 75 ° C.
See also
Individual evidence
- ↑ Data sheet cis-aconitic acid from AlfaAesar, accessed on May 8, 2010 ( PDF )(JavaScript required) .
- ↑ Data sheet trans-aconitic acid from AlfaAesar, accessed on May 8, 2010 ( PDF )(JavaScript required) .
- ↑ a b data sheet trans-aconitic acid (PDF) from Carl Roth , accessed on May 8, 2010.
- ↑ a b c d e f g h Entry on aconitic acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b data sheet cis-Aconitic acid from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ Bruce, WF: Aconitic acid In: Organic Syntheses . 17, 1937, p. 1, doi : 10.15227 / orgsyn.017.0001 ; Coll. Vol. 2, 1943, p. 12 ( PDF ).
- ↑ B. Pawolleck: substitution products of citric acid and an attempt for the synthesis of the latter. In: Justus Liebig's Annals of Chemistry. 178, 1875, p. 150, doi : 10.1002 / jlac.18751780203 .
- ↑ External identifiers or database links for cis-aconitic anhydride : CAS number: 6318-55-4, EC number: 228-663-3, ECHA InfoCard: 100.026.058 , PubChem : 65163 , Wikidata : Q55972704 .

![{\ displaystyle \ mathrm {C_ {6} H_ {8} O_ {7} \ {\ xrightarrow [{}] {H_ {2} SO_ {4}}} \ C_ {6} H_ {6} O_ {6} \ + \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/97821ad9295bf688aafbc92ccadb7d1773ec54af)