Acridon
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
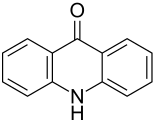
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Acridon | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 9 NO | ||||||||||||||||||
| Brief description |
white to yellowish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 195.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
360-362 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Acridone (9 (10 H ) -Acridone) is structurally related to its parent body acridine and the polycyclic aromatic hydrocarbon anthracene , but in contrast to this it has a heteroaromatic 4-pyridone as the central six-membered ring . The acridone structure can also be derived from the quinacridone structure by removing a 4-pyridone ring from it together with an adjoining peripheral ring.
history
According to a publication by Karl Drechsler , a student of Guido Goldschmiedt at the Imperial and Royal University of Vienna , Moriz Freund discovered the material in 1896 during experiments at the University of Prague . Drechsler was then able to produce the material in larger quantities and then examine it more closely.
use
Acridone derivatives can be used as fluorescence probes in molecular biology or to measure the Lewis acidity of metal ion salts. In medicine , acridone alkaloids are suitable as an active ingredient against cancer and malaria .
Individual evidence
- ↑ a b Acridon data sheet from Acros, accessed September 1, 2007.
- ↑ a b Acridone data sheet from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ↑ Karl Drechsler: About a base C 13 H 9 NO that is formed when aluminum chloride acts on o-nitrobenzyl chloride and benzene . In: Monthly magazine for chemistry. 35, 1914, p. 533, doi : 10.1007 / BF01519382 .
- ↑ JA Smith, RM West, M. Allen: Acridones and Quinacridones: Novel Fluorophores for Fluorescence Lifetime Studies , in: Journal of Fluorescence 2004 , 14 , pp. 151-171; doi : 10.1023 / B: JOFL.0000016287.56322.eb .
- ↑ SH Mihindukulasuriya, TK Morcone, LB McGown: Characterization of acridone dyes for use in four-decay detection in DNA sequencing , in: Electrophoresis 2003 , 24 , pp. 20-25; doi : 10.1002 / elps.200390017 .
- ↑ S. Fukuzumi, K. Ohkubo: Fluorescence Maxima of 10-Methylacridone-Metal Ion Salt Complexes: A Convenient and Quantitative Measure of Lewis Acidity of Metal Ion Salts , in: J. Am. Chem. Soc. 2002 , 124 , pp. 10270-10271; doi : 10.1021 / ja026613o .
- ↑ N. Guilbaud, S. Leonce, F. Tillequin, M. Koch, JA Hickman, A. Pierre: Acronycine derivatives as promising antitumor agents , in: Anti-Cancer Drugs 2002 , 13 , pp. 445-449.
- ↑ LK Basco, S. Mitaku, AL Skaltsounis, N. Ravelomanantsoa, F. Tillequin, M. Koch, J. Le Bras: In Vitro Activities of Furoquinoline and Acridone Alkaloids against Plasmodium falciparum , in: Antimicrob. Agents Chemother. 1994 , 38 , pp. 1169-1171; PMC 188171 (free full text).