4-pyridone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-pyridone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 5 NO | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 95.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
143-145 ° C |
|||||||||||||||
| boiling point |
230–235 ° C (16 hPa) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
4-pyridone is an organic compound with the empirical formula C 5 H 5 NO. She belongs to the group of Pyridons .
presentation
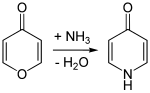
Analogously to the isomeric 2-pyridone , 4-pyridone can be obtained by reacting 4-pyrone with ammonia .
properties
The keto form 4-pyridone is in chemical equilibrium with the tautomeric hydroxo form 4-hydroxypyridine . In solution , the equilibrium is almost entirely on the side of the ketone. The hydroxoform only represents a significant proportion in equilibrium in highly dilute solutions and in very non-polar solvents. In the gas phase, however, the hydroxoform is the dominant species.
The reaction behavior of 4-pyridone is determined by the keto form, which is why the designation 4-pyridinol should be avoided, as it implies a reactivity similar to phenol , which however does not occur. In solution, 4-pyridone has no classic aromatic properties. Thus, the isomeric has 3-hydroxypyridine a pyridintypischen pK s value , while 4-pyridone a significantly lower basicity and having amidähnlich the oxygen protonated is.
4-Pyridone forms yellow-colored complexes with iron (III) chloride .
Occurrence and use
4-pyridone in free form does not occur naturally, but the basic structure occurs as a structural component in pyridosine , a hydrolysis product of milk . It also occurs in the natural substance mimopudine and the non- biogenic amino acid mimosine .
The compound serves as a raw material for the synthesis of 4-chloropyridine and 3-hydroxy-4-pyridones. The latter are used as aluminum and iron binding drugs .
Individual evidence
- ↑ a b c d e f Entry on pyridinoles. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c data sheet 4-pyridone at AlfaAesar, accessed on March 6, 2010 ( PDF )(JavaScript required) .
- ↑ a b Data sheet 4-Hydroxypyridine from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ External identifiers or database links for 4-hydroxypyridine : CAS number: 626-64-2, EC number: 210-958-3, ECHA InfoCard: 100.009.963 , Wikidata : Q24730939 .
- ↑ a b J.A. Joules, K. Mills: Heterocyclic Chemistry 2000 , 4th Edition, Blackwell Science, Oxford, pp. 88-91; ISBN 0-632-05453-0 .