Aluminum triisopropoxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Aluminum triisopropoxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 21 AlO 3 | |||||||||||||||
| Brief description |
white solid with an alcoholic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 204.25 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.035 g cm −3 |
|||||||||||||||
| Melting point |
130-134 ° C |
|||||||||||||||
| boiling point |
135 ° C at 13 hPa |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Aluminum triisopropoxide is a chemical compound of aluminum from the group of alcoholates .
Extraction and presentation
Aluminum triisopropoxide can be produced by reacting amalgamated aluminum with isopropyl alcohol (similar to the process with tert-butyl alcohol ).
properties
Aluminum triisopropoxide is a flammable, moisture-sensitive white solid with an alcoholic odor that decomposes in water. Aluminum triisopropoxide is hydrolyzed to aluminum oxide by moisture . It is a versatile oligomeric Lewis acid catalyst.
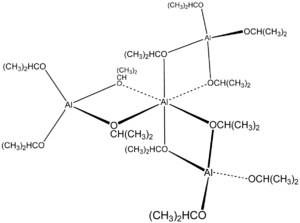
The compound has a tetrameric structure.
use
It is used as a specific reducing agent in the Meerwein-Ponndorf-Verley reduction or in a catalytic amount in the Oppenauer oxidation .
Individual evidence
- ↑ a b c d e f g h i j Entry on aluminum triisopropanolate in the GESTIS substance database of the IFA , accessed on September 13, 2015(JavaScript required) .
- ↑ Entry on aluminum triisopropanolate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Seung-Joon Yoo, Ho-Sung Yoon, Hee Dong Jang, Jung-Woon Lee, Seung-Tae Hong, Min-Jae Lee, Se-Il Lee, Ki-Won Jun: Synthesis of aluminum isopropoxide from aluminum dross. In: Korean Journal of Chemical Engineering. 23, 2006, p. 683, doi : 10.1007 / BF02706815 .
- ^ Wayne, W .; Adkins. H .: Aluminum tert-Butoxide In: Organic Syntheses . 21, 1941, p. 8, doi : 10.15227 / orgsyn.021.0008 ; Coll. Vol. 3, 1955, p. 48 ( PDF ).
- ↑ Data sheet Aluminum isopropoxide, ≥99.99% trace metals basis from Sigma-Aldrich , accessed on September 13, 2015 ( PDF ).
- ↑ Thomas A. Zevaco, Annette Janssen, Jakub Sypien, Eckhard Dinjus: Aluminum triisopropoxide: An inexpensive and easy-to-handle catalyst of the copolymerization of cyclohexene oxide with CO 2 . In: Green Chemistry. 7, 2005, p. 659, doi : 10.1039 / B504798F .
- ↑ Rene 'König: Local Structures of Nanoscopic Aluminum Alkoxide Fluorides And Chemically Related Crystalline Compounds . disserta Verlag, 2009, ISBN 978-3-942109-00-0 , p. 147 ( limited preview in Google Book search).
- ↑ Hans Wilhelm Bach, et al .: Houben-Weyl Methods of Organic Chemistry Vol. E 3, 4th Edition Supplement Aldehydes . Georg Thieme Verlag, 2014, ISBN 978-3-13-181134-9 , p. 274 ( limited preview in Google Book search).
