Aminobenzotrifluoride
| Aminobenzotrifluoride | ||||||||||||||
| Surname | 2-aminobenzotrifluoride | 3-aminobenzotrifluoride | 4-aminobenzotrifluoride | |||||||||||
| other names |
|
|
|
|||||||||||
| Structural formula |

|

|
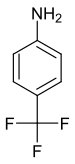
|
|||||||||||
| CAS number | 88-17-5 | 98-16-8 | 455-14-1 | |||||||||||
| ECHA InfoCard | 100,001,642 | 100.002.404 | 100.006.579 | |||||||||||
| PubChem | 6922 | 7375 | 9964 | |||||||||||
| Molecular formula | C 7 H 6 F 3 N | |||||||||||||
| Molar mass | 161.13 g mol −1 | |||||||||||||
| Physical state | liquid | |||||||||||||
| Brief description | yellow to brown liquids with an unpleasant amine-like odor | |||||||||||||
| Melting point | −33 ° C | 5 ° C | 3 ° C | |||||||||||
| boiling point | 171 ° C | 187 ° C | 83 ° C (16 hPa) | |||||||||||
| density | 1.29 g cm −3 (20 ° C) | 1.295 g cm −3 (20 ° C) | 1.295 g cm −3 (20 ° C) | |||||||||||
| Vapor pressure | 1.3 hPa (20 ° C) | 0.865 hPa (20 ° C) | 1 hPa (38 ° C) | |||||||||||
| solubility | poorly soluble in water (~ 5 g l −1 at 20 ° C) | |||||||||||||
| Refractive index | 1.481 (20 ° C) | 1.480 (20 ° C) | 1.483 (20 ° C) | |||||||||||
|
GHS labeling |
|
|
|
|||||||||||
| H and P phrases | 302-315-317-319-373-411 | 302-312-330-315-318-335-373-411 | 301-319-410 | |||||||||||
| 261-280-301 + 310-311 |
280-301 + 330 + 331-302 + 352 304 + 340-305 + 351 + 338-332 + 313-310 |
273-305 + 351 + 338-314 | ||||||||||||
| LD 50 | 480 mg kg −1 (oral rat) | 480 mg kg −1 (oral rat) | 128 mg kg −1 (oral rat) | |||||||||||
Aminobenzotrifluorides are chemical compounds from the group of halogenated benzene derivatives , which are derived from benzotrifluoride . Depending on the position of the amino group in relation to the trifluoromethyl group , there are three isomers .
Extraction and presentation
3-aminobenzotrifluoride can be synthesized from benzotrifluoride by nitration with nitric acid to 3-nitrobenzotrifluoride and its subsequent reduction (catalytic hydrogenation). The other two isomers are also formed to a lesser extent. By initially chlorinating benzotrifluoride (which later has to be reversed by catalytic hydrogenation) and thus blocking the 3-position, the yield of 2-aminobenzotrifluoride can be greatly increased.
properties
Aminobenzotrifluorides are yellow to brown, slightly volatile liquids with an unpleasant odor, which are sparingly water-soluble. They decompose when heated strongly in the presence of air, producing hydrogen fluoride , carbon monoxide , carbon dioxide and nitrous gases .
use
3-aminobenzotrifluoride is used as an intermediate in the manufacture of pesticides (e.g. fluometuron and flurochloridone ) and pharmaceuticals (e.g. flufenamic acid ). 4-aminobenzotrifluoride is also used as an intermediate in the manufacture of drugs (e.g. leflunomide or teriflunomide ).
safety instructions
The vapors of aminobenzotrifluoride can form an explosive mixture with air ( flash point 55–85 ° C, ignition temperature 600 ° C).
Individual evidence
- ↑ a b c d e f g h Entry on 2-aminobenzotrifluoride in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d e f g h i j k Entry on 3-aminobenzotrifluoride in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d e f g Entry on 4-aminobenzotrifluoride in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b Data sheet 4-aminobenzotrifluoride (PDF) from Merck , accessed on December 27, 2019.
- ↑ Data sheet 2-aminobenzotrifluoride from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ Data sheet 3-aminobenzotrifluoride from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ Data sheet 4-aminobenzotrifluoride from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ Data sheet 2-aminobenzotrifluoride (PDF) from Merck , accessed on December 27, 2019.
- ↑ Alain Tressaud: Fluorine and the Environment ; ISBN 978-0-44452672-4 , p. 125 ( limited preview in Google book search).
- ↑ Ronald Eric Banks: Fluorine in agriculture ; ISBN 978-1-85957033-3 .
- ↑ Paul Knochel: Modern solvents in organic synthesis ; ISBN 978-3-540-66213-6 , p. 84.
- ^ A process for preparing Teriflunomide .





