Flufenamic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Flufenamic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
Solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| density |
1.47 g cm −3 (flufenamic acid) |
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| Vapor pressure |
0.0038 Torr (100.5 ° C, flufenamic acid) |
|||||||||||||||||||||
| solubility |
0.0265 g l −1 (37 ° C, in water, flufenamic acid) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Flufenamic acid is a non-steroidal anti-inflammatory drug and non-opioid analgesic from the group of anthranilic acid derivatives. It is contained in ointments and is used for joint inflammation and muscle strains. The drug inhibits cyclooxygenase and hyaluronidase . The expression of COX-II is also throttled via NFkB . It could be shown that flufenamic acid unselectively inhibits cation channels . The mean elimination half-life is 9 hours, the extra renal elimination percentage is high.
history
In the 1950s, the Parke Davis company (USA), headed by Andrew C. Bratton Jr., began the search for a new anti-inflammatory agent. The synthesis was carried out under the direction of Eldon M. Jones (1914-1996). However, Robert A. Scherrer is considered to be the discoverer of flufenamic acid. In 1961, Parke Davis first applied for a patent for flufenamic acid in France.
Presentation and extraction
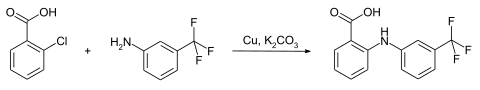
The synthesis of flufenamic acid is carried out by the reaction in an Ullmann-Goldberg reaction of 2-chlorobenzoic acid with 3-trifluoromethylaniline in the presence of copper and a base.
Instead of 2-chlorobenzoic acid, it is also possible to start from the bromine or iodine derivative.
properties
Flufenamic acid shows a pronounced tendency to develop polymorphic forms. Eight different crystal forms could be identified by means of thermoanalytical and IR spectroscopic investigations. The melting points of the various polymorphic forms are 134 ° C (Form I), 128 ° C (Form II), 126 ° C (Form III), 124 ° C (Form IV), 122 ° C (Form V), 120 ° C (Form VI), 118 ° C (Form VII) and 108 ± 5 ° C (Form VIII). The enthalpies of fusion of three forms are known to be 27.7 kJ mol −1 (form I), 28.9 kJ mol −1 and 23.4 kJ mol −1 (form V). However, only forms I and III are of practical relevance, since only these can be obtained via solvent crystallization. These two forms form an enantiotropic system with a transition point at 42 ° C. Below this temperature and thus at room temperature, form III is the thermodynamically stable form and form I is metastable. The relationship is reversed above the transition point. Form I becomes stable and form III becomes metastable. Both crystal forms form a monoclinic crystal lattice. Both crystal lattices differ with regard to the geometric arrangement of the trifluoromethylphenyl function to the benzoic acid function and the hydrogen bonds that are formed . The solubility in water at 37 ° C is only low at 0.0265 g · l −1 . Better solubilities are achieved at 37 ° C in propylene glycol with 2.239 g · l −1 , in ethanol with 6.918 g · l −1 and in chloroform with 19.055 g · l −1 . In a 1: 1 mixture of water and ethanol, the solubility at 37 ° C is 0.224 g · l −1 . With an acid constant pK s of 3.85, the compound is a moderately strong acid.
Trade names
Algesalona (D), Dignodolin (D), Mobilisin (D, I), Mobilat (D), Rheuma Lindofluid (D), Arlef (F, GB), Meralen (GB), Assan Emgel (CH), Assan Gel (CH )
Individual evidence
- ↑ a b c M. Kuhnert-Brandstätter, L. Borka, G. Friedrich-Sander: On the polymorphism of drugs: Flufenamic acid and BL 191. In: Arch. Pharm. 307 (1974), pp. 845-853.
- ↑ a b H. M. Murthy, Krishna, TN Bhat, M. Vijayan: Structure of a new crystal form of 2 - {[3- (trifluoromethyl) phenyl] amino} benzoic acid (flufenamic acid). In: Acta Cryst. B: Struct. Cryst. Cryst. Chem. 38 (1982), pp. 315-317, doi: 10.1107 / S0567740882002763 .
- ↑ a b c d e A. Burger, R. Ramberger: Thermodynamic relationships between polymorphic modifications: flufenamic acid and mefenamic acid. In: Microchim. Acta. 73 (1980), pp. 17-28, doi: 10.1007 / BF01197228 .
- ^ T. Konno: Physical and Chemical Changes of Medicinicals in Mixtures with Adsorbents in the Solid State. III. Determination of Vapor Pressure of Solid Drugs by Steam Distillation. In: Chem. Pharm. Bull. 38 (1990), pp. 1032-1034, doi : 10.1248 / cpb.38.1032 , pdf .
- ^ A b J. Priborski, K. Takayama, Y. Obata, Z. Priborska, T. Nagai: Influence of Limonene and Laurocamram on Percutaneous Adsorption of Nonsteroidal Anti-Infammatory Drugs. In: Arzneimittel.-Forsch./Drug Res. 42 (1992), pp. 116-119.
- ↑ a b Flufenamic acid data sheet from Sigma-Aldrich , accessed on May 18, 2017 ( PDF ).
- ^ A b c d A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. (2000), Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 .
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 .
- ↑ RB Moffett, BD Aspergren: Aminoalkylphenothiazines. In: J. Am. Chem. Soc. 82 (1960), pp. 1600-1607, doi: 10.1021 / ja01492a022 .
- ^ R. Carrasco, RF Pellon, J. Elguero, P. Goya, JA Paez: The Use of Ultrasound in the Synthesis of N-Thranilic Acids by the Ullmann Goldberg Reaction. In: Synth. Comm. 19 (1989), pp. 2077-2080, doi: 10.1080 / 00397918908052600 .
- ↑ JS Kaltenbronn, RA Scherrer, FW Short, EM Jones, HR Beatty: Medic. Forsch./Drug Res. 33 (1983), pp. 621-627.
- ↑ JH Wilkinson, IL Finar: A study of the properties of fluorine-substituiertem 5-aminoacridines and related compounds. Part II: 5-Amino-2-and -4-trifluoromethyl acridines. In: J. Chem. Soc. . 1948, pp. 32-35, doi: 10.1039 / JR9480000032 .
- ^ A b X. Chen, T. Li, KR Morris, SR Byrn: Crystal Packing and Chemical Reactivity of Two Polymorphs of Flufenamic Acid with Ammonia. In: Mol. Cryst. Liq. Cryst. . 381 (2002), pp. 121-131.
- ↑ H. Terada, S. Muraoka, T. Fujita: Structure-Activity Relationship of Fenamic Acids. In: J. Med. Chem. . 1974, 17, pp. 330-334.