Androstenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
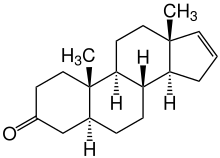
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Androstenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 19 H 28 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 272.43 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
140-144 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Androstenone is a steroid ( androgen ) and a metabolite of the sex hormone testosterone , which is used as a pheromone in various mammals and is perceived via the vomeronasal organ . The function of androstenone as a pheromone is also discussed in humans, but has not yet been clearly clarified.
Androstenone is formed in the Leydig cells in the testes and migrates through the adipose tissue to the salivary glands at sexual maturity , where the substance is released into the air via saliva.
Androstenone in the sow
Androstenone is - together with skatole - responsible for boar taint of the boar . In the intoxicated sow it induces the rigor of tolerance . Boar smell in meat products occurs only in about every tenth animal and only in cooked meat products, but not in raw meat and sausage products. The boar , followed by the slaughter androstenone sample, therefore, provides an alternative to piglet castration is. (See. Also pig production ).
An alternative to piglet castration and boar fattening is immunocastration , which in Europe has so far only been used to a limited extent in Belgium (as of 2016).
Individual evidence
- ↑ a b c d data sheet 5α-Androst-16-en-3-one from Sigma-Aldrich , accessed on February 1, 2013 ( PDF ).
- ^ R. Claus, W. Alsing: J. Endocr. 68 (1976) 483.
- ↑ J. Havlicek, et al .: Current issues in the study of androstenes in human chemosignaling In: Vitamines and Hormones: Pheromones 83 (2010), pp. 47-81.
- ^ V. Prelog , L. Ruzicka: Helvetica Chimica Acta 27 (1944) 61.
- ^ V. Prelog, L. Ruzicka: Helvetica Chimica Acta 27 (1944) 66.
- ↑ Manhood does not have to harm the flesh . In: Neue Zürcher Zeitung , March 3, 2010.