Bromacil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bromacil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 13 BrN 2 O 2 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 261.12 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.55 g cm −3 |
|||||||||||||||
| Melting point |
157.5-160 ° C |
|||||||||||||||
| solubility |
very heavy in water (815 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 1 ml m −3 or 10 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bromacil is a chemical compound from the group of pyrimidinediones or uracils .
Extraction and presentation
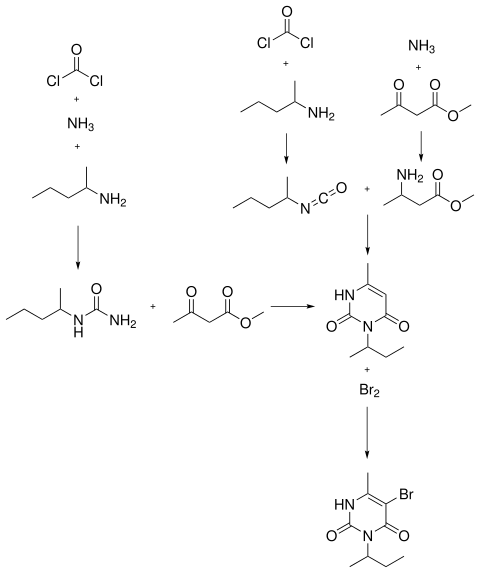
Bromacil can be produced by reacting isopropyl isocyanate with methyl 3-aminocrotonate and subsequent cyclization in sodium hydroxide solution at elevated temperature and bromination in acetic acid.
Alternatively, it can be prepared by reacting phosgene and ammonia with sec -butylamine to form sec- butylurea , which in turn is reacted with methyl acetoacetate to form 3- sec- butyl-6-methyluracil. Subsequent bromination produces bromacil.
Stereochemistry
Generally speaking, chemical compounds with at least one stereocenter form up to 2 n stereoisomers . Here n is the number of stereocenters. Accordingly, there are two stereoisomers in Bromacil, which have also been confirmed experimentally:
| Enantiomers of bromacil | |
|---|---|
 CAS number: 154799-70-9 |
 CAS number: 154799-66-3 |
properties
Bromacil is a non-flammable, colorless and odorless solid that is very sparingly soluble in water. It decomposes when heated. The compound is stable to hydrolysis and sunlight.
use
Bromacil is used as a herbicide . It is used to control annual and perennial grasses and broad-leaved weeds. The effect is based on the inhibition of photosynthesis.
Admission
Bromacil was approved in the FRG between 1971 and 1990.
In 2002 the substance was not included in the list of plant protection active ingredients approved in the European Union . Plant protection products containing the active ingredient bromacil are not permitted in any EU country, not even in Switzerland.
Web links
Individual evidence
- ↑ a b c d e f g h i j k Entry on Bromacil in the GESTIS substance database of the IFA , accessed on March 1, 2013(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for Bromacil ), accessed on March 4, 2020.
- ↑ a b Entry on Bromacil in the Hazardous Substances Data Bank , accessed March 1, 2013.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-08-095716-1 , pp. 568 ( limited preview in Google Book search).
- ↑ Paula Y. Bruice: Organic Chemistry: Study compact . Pearson Studium, Munich 2011, ISBN 978-3-86894-102-9 , p. 205.
- ↑ Elim M. Ulrich, Candice M. Morrison, Michael M. Goldsmith, William T. Foremann: Chiral Pesticides: Identification, Description, and Environmental Implications . In: Reviews of Environmental Contaminations and Toxicology. Springer 2012, Boston, Volume 217, pp. 1–74, DOI: 10.1007 / 978-1-4014-2329-4_1 , see p. 20.
- ^ Terence Robert Roberts, David Herd Hutson: Metabolic Pathways of Agrochemicals. Part 1. Herbicides and Plant Growth Regulators . Royal Society of Chemistry, 1998, ISBN 0-85404-494-9 , pp. 696 ( limited preview in Google Book search).
- ^ Stanley A. Greene: Sittig's Handbook of Pesticides and Agricultural Chemicals . William Andrew, 2005, ISBN 0-8155-1903-6 , pp. 1039 ( limited preview in Google Book search).
- ^ Müfit Bahadir, Harun Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 3-642-56998-6 , pp. 236 ( limited preview in Google Book search).
- ↑ Peter Brandt: Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products , Approval History and Regulations of the Plant Protection Application Ordinance . Springer, 2010, ISBN 3-0348-0029-0 , pp. 10 ( limited preview in Google Book search).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Bromacil in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 24, 2016.