Bromoethanols
| Bromoethanols | |||||||
| Surname | 2-bromoethanol | 2,2-dibromoethanol | 2,2,2-tribromoethanol | ||||
| other names | Ethylene bromohydrin | Avertin ® | |||||
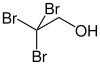
| Structural formula |  |
|
|||||
| CAS number | 540-51-2 | 83206-47-7 | 75-80-9 | ||||
| PubChem | 10898 | 123582 | 6400 | ||||
| Molecular formula | C 2 H 5 BrO | C 2 H 4 Br 2 O | C 2 H 3 Br 3 O | ||||
| Molar mass | 124.97 g mol −1 | 203.86 g mol −1 | 282.76 g mol −1 | ||||
| Physical state | liquid | firmly | |||||
| description | brown clear liquid | colorless liquid | white crystals | ||||
| Melting point | −80 ° C | 73-79 ° C | |||||
| boiling point | 149–150 ° C 56–57 ° C (27 hPa) |
179-181 ° C | 92–93 ° C (13 hPa) | ||||
| density | 1.763 g cm −3 (25 ° C) | 2.35 g cm −3 (0 ° C) | |||||
| Vapor pressure | 2.4 mmHg (20 ° C) | ||||||
| solubility | soluble in water | ||||||
| Refractive index | 1.492 | ||||||
|
GHS labeling |
|
|
|
||||
| H and P phrases | 301 + 311 + 331-314 | see above | 302-315-319-335 | ||||
| no EUH phrases | no EUH phrases | no EUH phrases | |||||
| 261-280-301 + 310-305 + 351 + 338-310 | see above | 261-305 + 351 + 338 | |||||
| LD 50 | 930 mg / kg (oral, mouse) | ||||||
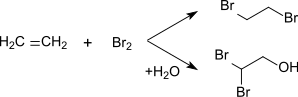
Bromoethanols is the collective term for three organic chemical compounds that are derived from ethanol , with one or more hydrogen atoms being replaced by bromine atoms .
presentation
Bromoethanol
2-Bromoethanol can be produced by introducing ethylene into bromine water .
2-Bromoethanol can be made from ethylene oxide and hydrogen bromide .
From ethylene and hydrogen bromide or phosphorus which is synthesis possible.
The addition of hypobromous acid to ethylene also produces 2-bromoethanol.
Dibromoethanol
2,2-Dibromoethanol is formed by adding hypobromous acid to bromoethene .
It is also a by-product of the bromination of ethene in aqueous solution.
Tribromoethanol
2,2,2-tribromoethanol is by reaction of aluminum ethoxide and elemental prepared bromine.
properties
Bromoethanol
Ethylene oxide is formed from 2-bromoethanol and potassium hydroxide through an intramolecular condensation reaction .
It reacts further with hydrogen bromide to form 1,2-dibromoethane .
Dibromoethanol
From 2,2-dibromoethanol, 1,1-dibromoethene is formed by elimination of water .
With potassium hydroxide, 2-bromoxirane is formed .
use
Bromoethanol
2-Bromoethanol is used as a reagent in organic syntheses when carboxylic acid groups are to be protected as 2-bromoethyl esters .
Tribromoethanol
Tribromoethanol is used under the name Avertin ® as an anesthetic in small animal medicine , especially in mice .
Individual evidence
- ↑ a b c d e f data sheet 2-bromoethanol from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b c d e f g E. Demole: "About the substitution derivatives of ethylene oxide" in reports of the German Chemical Society 1876 , 9 , pp. 45-51. Full text
- ^ A b W. Erhardt: "Anesthesia and analgesia in small and domestic animals as well as in birds, reptiles, amphibians and fish", Schattauer Verlag, 2004. ISBN 9783794520572 . P. 48. ( limited preview in Google Book search)
- ↑ a b c data sheet bromoethanols at AlfaAesar, accessed on April 13, 2011 ( PDF )(JavaScript required) .
- ↑ a b c data sheet 2,2,2-tribromoethanol from Sigma-Aldrich , accessed on April 13, 2011 ( PDF ).
- ↑ Entry on 2-bromoethanol at ChemBlink , accessed on April 13, 2011.
- ↑ Data sheet 2,2,2-tribromoethanol from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ^ A b F. K. Thayer, CS Marvel, GS Hiers: 2-Bromoethanol In: Organic Syntheses . 6, 1926, p. 12, doi : 10.15227 / orgsyn.006.0012 ; Coll. Vol. 1, 1941, p. 117 ( PDF ).
- ^ OD Tyagi, M. Yadav: "A textbook of organic reaction mechanism", p. 212, ISBN 9788170413974 . ( limited preview in Google Book search)
- ^ A. Kar: "Medicinal Chemistry", Verlag New Age International, 2005, p. 66, ISBN 9788122415650 . ( limited preview in Google Book search)
- ↑ Entry at www.chemsink.com