Calcium acetylacetonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
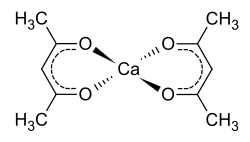
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium acetylacetonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 14 CaO 4 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 238.3 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
175–280 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in water (11.9 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium acetylacetonate is a chemical compound of calcium from the group of substituted alcoholates and a derivative of acetylacetone .
Extraction and presentation
Calcium acetylacetonate can be obtained by reacting calcium chloride or calcium hydroxide with acetylacetone in sodium hydroxide solution .
properties
Calcium acetylacetonate is a flammable, hardly inflammable, not very volatile, white, odorless solid that is not very soluble in water. It decomposes when heated above> 280 ° C. Its aqueous solution has an alkaline reaction.
use
Calcium acetylacetonate, like magnesium acetylacetonate and zinc acetylacetonate, is used as a stabilizer in PVC . It is also used as a catalyst.
Risk assessment
Calcium acetylacetonate was included in the EU's ongoing action plan ( CoRAP ) in 2015 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The uptake of calcium acetylacetonate was caused by concerns about worker exposure , high (aggregated) tonnage and other hazard-related concerns, as well as the suspected hazards from sensitizing properties. The re-evaluation has been running since 2015 and is carried out by Germany .
Individual evidence
- ↑ a b c d e f g h Entry on calcium acetylacetonate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 55 ( limited preview in Google Book search).
- ↑ Patent CN102898294 : Preparation method for calcium acetylacetonate. Filed September 11, 2012 , published January 30, 2013 , applicant: AnHui JiaXian Functional Auxiliary Co., Ltd., Inventor: 李兑, 黄先胜, 李平, 王艳, 陈新华.
- ↑ Study on Synthesis of Calcium Acetylacetonate - “Plastics Additives” 2013 年 01 期. en.cnki.com.cn, accessed July 11, 2016 .
- ↑ Michael Schiller: PVC Additives Performance, Chemistry, Developments, and Sustainability . Carl Hanser Verlag GmbH Co KG, 2015, ISBN 978-1-56990-544-9 , pp. 39 ( limited preview in Google Book search).
- ↑ Patent DE69817348T2 : Coated calcium or magnesium acetylacetonate, and their use as a stabilizer for halogenated polymers. Registered on June 4, 1998 , published on July 1, 2004 , applicant: Rhodia Chimie, inventor: Michel Gay, Françoise Henrio.
- ^ Emo Chiellini, International Center of Biopolymer Technology: Biorelated Polymers Sustainable Polymer Science and Technology . Springer Science & Business Media, 2001, ISBN 978-0-306-46652-6 , pp. 189 ( limited preview in Google Book search).
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): bis (pentane-2,4-dionato) calcium , accessed on March 26, 2019.

