Calixarenes
Calixarenes ( Calix = Latin for "cup"; related to the German word) are a class of organic macrocyclic compounds . Calixarenes consist of phenol units which are each connected with methylene bridges in the ortho position to the OH group. The number of monomers contained is given as [n] in calix [n] arenes. The most common are tetramers made up of 4 phenolic units; these are therefore called calix [4] arenes. But also pentamers (calix [5] arenes), hexamers (calix [6] arenes) and octamers (calix [8] arenes) are relatively easy to access by varying the reaction conditions (temperature, solvent, etc.) in so-called one - pot reactions . Numbers other than 4, 5, 6 or 8 phenol units are also possible, but have so far remained meaningless since their specific synthesis is too expensive.
synthesis
Calixarenes can be synthesized from phenols and formaldehyde with acid and base catalysis . Phenols which are substituted in the para position are preferably used in order to prevent polymerization . In addition to the “classic” phenol calixarenes, there are also calixarenes made from resorcinol (so-called resorcinarenes) or pyrogallol with long-chain aldehydes . The reaction that takes place is an electrophilic aromatic substitution with subsequent elimination of water. Special calixarenes can also be obtained by fragment condensation or the construction of a linear precursor molecule with a subsequent ring closure reaction.
structure
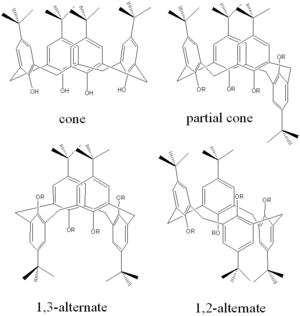
In the case of the calixarenes, different geometric molecular structures are possible due to the hindered rotation around the methylene bridges. The vase-like conformation ( cone structure, point group C 4v ) can be stabilized by bulky substituents . In addition to this structure, there are three other conformations, partial cone (C S ), 1,2-alternate (C 2h ) and 1,3-alternate (D 2d ). In the case of syntheses with calixarenes, there is sometimes a conformational change; this can in certain cases be suppressed by the choice of reaction conditions or, if desired, can also be brought about in a targeted manner.
history
In 1902 Leo Baekeland first produced a resin from phenol and formaldehyde, Bakelite . The three possible ways of attacking the phenol in the ortho- and para- positions resulted in a three-dimensional network. It was not until 1942 that Alois Zinke and Erich Ziegler attempted a controlled synthesis of the polymer with p-tert- butylphenol and formaldehyde. They received a crystalline substance, but due to the possibilities at the time, they could only lower the melting point to determine the molecular mass. Only John Cornforth received a crystal structure analysis of these substances with the help of Dorothy Mary Hodgkin .
In 1975 the name “Calixaren” was recommended for these substance classes by David Gutsche , and it gradually established itself for related bodies (e.g. Resorcinarene). Gutsche also recognized the instability of the cone conformation in unsubstituted calixarenes and Donald J. Cram managed to stabilize unsubstituted calixarenes in the cone conformation using carbonic acid esters .
Applications
Most of the syntheses known from the chemistry of phenols can in principle also be applied to calixarenes, but one must take into account the special molecular geometry, which sometimes prevents certain reagents from attacking. Calixarenes can be esterified and etherified at the phenolic OH group; Various substitutions are possible on the C atom in the para position. Calixarenes serve as it were as a molecular skeleton for various chelate - complexing and sensors for sodium ions . The selectivity for cadmium , lead , lanthanoids and actinides can be increased by functionalization. In addition, calixarenes can be used in stationary phases in HPLC or electron beam lithography . Calixarenes also catalyze various reactions; so z. B. the reaction rate in the Menschutkin reaction between quinuclidine and 1-butyl bromide increased by a factor of 1600.
Calixarenes are also of interest as potential drugs , diagnostics or drug carriers for pharmaceutical applications . Calixarenes modified with the help of cyclic peptide groups have antibody- like properties when binding proteins and can be used as antibody mimetics .
literature
- ^ Gutsche, C. David (1989). Calixarenes . Cambridge: Royal Society of Chemistry.
- ^ Byron W. Purse, Arnaud Gissot, Julius Rebek: A Deep Cavitand Provides a Structured Environment for the Menschutkin Reaction . In: Journal of the American Chemical Society . tape 127 , no. 32 , 2005, pp. 11222-11223 , doi : 10.1021 / ja052877 + .
- ↑ Yoshitomo Hamuro, Mercedes Crego Calama, Hyung Soon Park, Andrew D. Hamilton: A Calixarene with Four Peptide Loops: An Antibody Mimic for Recognition of Protein Surfaces . In: Angew. Chem. Int. Ed. Engl. Volume 36 , no. 23 , 1997, doi : 10.1002 / anie.199726801 .