Camps quinoline synthesis
The Camps quinoline synthesis was first discovered by Icilio Guareschi (1847–1918) in 1894. Guareschi had ortho- amino acetophenone react with ethyl cyanoacetate in a condensation reaction to form 2- hydroxy -3- cyano -4-methylquinoline. Rudolf Camps then expanded this reaction to synthesize hydroxy quinolines by reacting N - acyl - ortho- acyl aniline with a base . The reaction is sometimes referred to as the Camps reaction or Camps cyclization .
Overview
N -acyl- ortho -acylanilines react in the presence of a base to form 4-hydroxyquinolines (middle) and 2-hydroxyquinolines:
 R 1 and R 2 are organic radicals or hydrogen , it being possible for the radicals to be identical or different.
R 1 and R 2 are organic radicals or hydrogen , it being possible for the radicals to be identical or different.
The reaction can therefore be carried out, for example, with ortho- amino derivatives of acetophenone , benzophenone , benzyl acetate or propiophenone and leads to a mixture of the two products. If one of the radicals has electron-withdrawing properties (e.g. cyano , acyl or phenyl group ), only one product is created:
- R 1 is an electron withdrawing group → 4-hydroxyquinolines (middle)
- R 2 is an electron withdrawing group → 2-hydroxyquinolines (right)
mechanism
The following describes the two different reaction mechanisms that take place depending on the location of the deprotonation. Here, too, R 1 and R 2 are organic radicals or hydrogen.
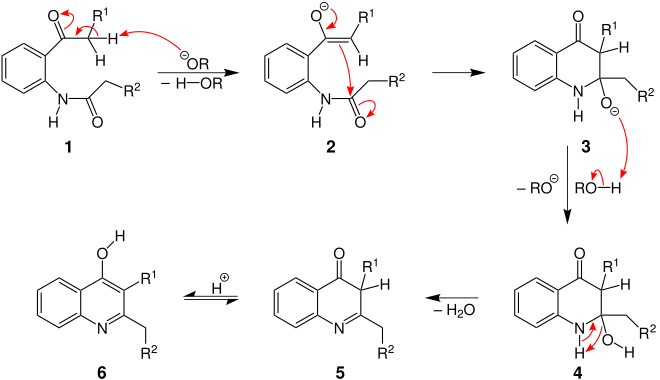
Formation of 2,3-dialkyl-4-hydroxyquinoline
This product is formed during the deprotonation of the methylene group on the R 1 residue, i.e. when R 1 is an electron-withdrawing residue or a mixture of the two products is formed:
First, the base deprotonates the methylene group of the N -acyl- ortho- acylaniline 1 , whereby the enolate 2 is formed. With the re-formation of the carbonyl group , the C = C double bond just formed attacks the carbon atom of the other carbonyl group nucleophilically and thus forms a heterocyclic six-membered ring 3 . The resulting alcoholate 3 is protonated by alcohol to form molecule 4 and then splits off water. In the ketone 5 then finds a tautomerization instead, resulting in the formation of the 2,3-dialkyl-4-hydroxyquinoline 6 leads.
Formation of 3,4-dialkyl-2-hydroxyquinoline
The 3,4-dialkyl-2-hydroxyquinoline, on the other hand, is formed when the methylene group of the amino-acyl group is deprotonated (R 2 is an electron-withdrawing radical or as part of the product mixture):
Here the methylene group on nitrogen 1 is deprotonated by the base. Here, too, an alcoholate is formed 7 . When a carbonyl group is formed, the double bond 7 that is formed attacks the carbon atom of the acyl group and forms a heterocyclic six-membered ring 8 . The resulting alcoholate group 8 is protonated by an alcohol 9 . A double bond is then formed with elimination of water . Here, too, a keto-enol tautomerism from ketone 10 to 3,4-dialkyl-2-hydroxyquinoline 11 finally takes place.
See also
Individual evidence
- ↑ a b c d e f Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 598-602.
- ↑ CP Jones, KW Anderson, SL Buchwald: Sequential Cu-Catalyzed Amidation-Base-Mediated Camps Cyclization: A Two-Step Synthesis of 2-Aryl-4-quinolones from o -Halophenones . In: J. Org. Chem. Band 72 , no. 21 , 2007, p. 7968-7973 , doi : 10.1021 / jo701384n .
- ↑ R. Camps: Synthesis of α- and γ-Oxychinolinen . In: Archives of Pharmacy . tape 237 , no. 9 , 1899, pp. 659-691 , doi : 10.1002 / ardp.18992370902 .
- ↑ R. Camps: Synthesis of α- and γ-Oxychinolinen . In: Reports of the German Chemical Society . tape 32 , no. 3 , 1899, pp. 3228-3234 , doi : 10.1002 / cber.18990320389 .
- ↑ R. Camps: Synthesis of α- and γ-Oxychinolinen . In: Archives of Pharmacy . tape 239 , no. 8 , 1901, pp. 591-610 , doi : 10.1002 / ardp.19012390805 .
- ↑ R. Camps: From amidophenylpropiolic acid to kynurenic acid and its relatives . In: Reports of the German Chemical Society . tape 34 , no. 2 , 1901, p. 2703-2718 , doi : 10.1002 / cber.190103402221 .
- ↑ R. Camps: Synthesis of α- and γ-Oxychinolinen . In: Archives of Pharmacy . tape 240 , no. 2 , 1902, pp. 135-146 , doi : 10.1002 / ardp.19022400204 .
- ^ RH Manske: The Chemistry of Quinolines . In: Chem. Rev. Band 30 , no. 1 , 1942, p. 113-144 , doi : 10.1021 / cr60095a006 .
- ↑ CP Jones, KW Anderson, SL Buchwald: Sequential Cu-Catalyzed Amidation-Base-Mediated Camps Cyclization: A Two-Step Synthesis of 2-Aryl-4-quinolones from o -Halophenones . In: J. Org. Chem. Band 72 , no. 21 , 2007, p. 7968-7973 , doi : 10.1021 / jo701384n .
- ↑ a b c J. J. Li: Name Reactions. A Collection of Detailed Reaction Mechanisms . 3rd expanded edition, Springer, Berlin / Heidelberg 2006, ISBN 978-3-540-30030-4 , pp. 104-106.