Benzyl acetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
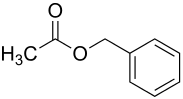
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzyl acetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 10 O 2 | |||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 150.18 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.06 g cm −3 |
|||||||||||||||
| Melting point |
−51 ° C |
|||||||||||||||
| boiling point |
214 ° C |
|||||||||||||||
| Vapor pressure |
0.19 mbar (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.5232 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzyl acetate is a chemical compound from the group of carboxylic acid esters .
Occurrence
Benzyl acetate occurs naturally in many fruits, mushrooms and especially flowers and their oils (for example jasmine oil and ylang-ylang oil ).
Extraction and presentation
Benzyl acetate can be obtained by reacting benzyl alcohol with acetic acid and sodium acetate or benzyl chloride with alkali acetates .
use
Because of its flowery smell, benzyl acetate is mainly used as a fragrance and also as a solvent (e.g. for cellulose acetates and nitrates, oils, lacquers, polishes and inks) and is contained in printing inks, lacquers and leveling agents in coating materials.
safety instructions
The vapors of benzyl acetate can form an explosive mixture with air ( flash point 102 ° C, ignition temperature 460 ° C).
Web links
- Joint FAO / WHO Expert Committee on Food Additives (JECFA), Monograph for BENZYL ACETATE, BENZYL ALCOHOL, BENZALDEHYDE, AND BENZOIC ACID AND ITS SALTS
- Process for the production of benzyl acetate and benzyl alcohol (patent-de)
Individual evidence
- ↑ a b c d e f g h i Entry on benzyl acetate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Data sheet acetic acid benzyl ester (PDF) from Merck , accessed on February 23, 2010.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-42.
- ↑ International Agency for Research on Cancer (IARC): Benzyl Acetate .
- ↑ Entry on benzyl acetate. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.