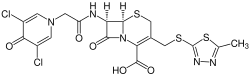
Cefacedon
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cefacedon | |||||||||||||||||||||
| other names |
7- {2- [3,5-dichloro-4-oxo-1 (4 H ) -pyridyl] -acetamido} -3- (5-methyl-1,3,4-thiadiazol-2-ylthiomethyl) -3- cephem-4-carboxylic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 18 H 15 Cl 2 N 5 O 5 S 3 | |||||||||||||||||||||
| Brief description |
white to yellowish white powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Disturbance of cell wall synthesis |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 548.43 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cefacedon is an antibiotic that is used to treat bacterial infections. It is produced semisynthetically and belongs to the class of cephalosporins of the 1st generation.
Cefazedon was first patented by Merck in 1975 . Medicines containing cefacedone (trade name Refosporin ) are no longer available in Germany.
indication
Cefazedon is indicated for the treatment of infections caused by sensitive pathogens (infections of the respiratory tract, urinary tract and biliary tract).
Working principle
The cefacedon molecules bind - like all cephalosporins - to specific penicillin-binding proteins , which are required to rebuild the bacterial cell wall. This prevents further synthesis of the bacterial cell wall.
Application
Cefazedon can be administered orally as well as intramuscularly and intravenously .
Individual evidence
- ↑ a b c d CEFAZEDONE. chemicalland21.com, accessed May 20, 2019 .
- ↑ a b bldpharm: SDS Cefazedon , accessed on December 27, 2019.
- ↑ a b Entry on Cefazedon. In: Römpp Online . Georg Thieme Verlag, accessed on May 22, 2019.
- ↑ Drug Update: Fluoroquinolones. German STI Society - Society for the Promotion of Sexual Health, accessed on May 20, 2019 .
- ↑ a b Conference Guide of the North Rhine-Westphalian Society for Urology, May 1981 , accessed on May 22, 2019.