Cerium (III) sulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
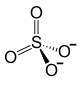
|
||||||||||
| General | ||||||||||
| Surname | Cerium (III) sulfate | |||||||||
| other names |
|
|||||||||
| Molecular formula | Ce 2 (SO 4 ) 3 | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 568.42 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
920 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cerium (III) sulfate is an inorganic chemical compound of cerium from the group of sulfates .
Extraction and presentation
Cerium (III) sulfate can be obtained by reacting cerium (IV) sulfate with oxalic acid , hydrazine or sodium thiosulfate .
It can also be produced by reacting cerium (IV) oxide with sulfur dioxide .
properties
Cerium (III) sulphate (anhydrous) is a hygroscopic white solid that begins to decompose above 600 ° C. It has a monoclinic crystal structure . Its tetrahydrate is a white solid that gives off its water of crystallization from 220 ° C. Like the white octahydrate, it has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . The nonahydrate has a hexagonal crystal structure with the space group P 6 3 / m (space group no. 176) . Hydrates with 12, 9, 8, 5, 4 and 2 parts of water of crystallization are known of the compound. The compound is one of the few compounds whose solubility in water decreases with temperature. The solubility of the anhydrate in water at 0 ° C is 101 g / l, at 100 ° C only 2.5 g / l, that of the octahydrate at 94.3 g / l at 20 ° C, 57 g / l at 40 ° C and 40.4 g / l at 60 ° C.
Individual evidence
- ↑ a b c d e data sheet Cerium (III) sulfate, ≥99.99% trace metals basis from Sigma-Aldrich , accessed on October 29, 2016 ( PDF ).
- ↑ a b c d e f g R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 374 ( limited preview in Google Book search).
- ↑ a b c Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , pp. 6 ( limited preview in Google Book search).
- ↑ G. Singh: Chemistry Of Lanthanides And Actinides . Discovery Publishing House, 2007, ISBN 978-81-8356-241-6 , pp. 262 ( limited preview in Google Book search).
- ^ Gunther Kolb: Fuel Processing For Fuel Cells . John Wiley & Sons, 2008, ISBN 3-527-31581-0 , pp. 103 ( limited preview in Google Book search).
- ↑ Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 978-0-412-30120-9 , pp. 2824 ( limited preview in Google Book search).
- ↑ Barbara M. Casari, Vratislav Langer: New Structure Type among Octahydrated Rare-Earth Sulfates, β-Ce2 (SO4) 3-8H2O, and a new Ce2 (SO4) 3-4H2O Polymorph. In: Journal of Inorganic and General Chemistry. 633, 2007, p. 1074, doi : 10.1002 / zaac.200700003 .
- ↑ T. Mioduski: Identification of saturating Solid Phases in the system Ce 2 (SO 4) 3-H2O from the Solubility Data. In: Journal of Thermal Analysis and Calorimetry. 55, p. 751, doi : 10.1023 / A: 1010161212184 .
- ^ Daniel L. Reger, Scott R. Goode, David W. Ball: Chemistry: Principles and Practice . Cengage Learning, 2009, ISBN 978-0-534-42012-3 , pp. 482 ( limited preview in Google Book search).

![\ mathrm {\ \! \ \ Biggr] _2} \ mathrm {\ \ Biggl [}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bafeab07e1d5bb8bb77e0767e55563945c55cd72)
![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)




