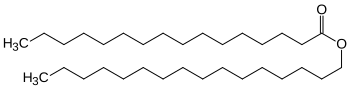
Cetyl palmitate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cetyl palmitate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 32 H 64 O 2 | |||||||||||||||
| Brief description |
almost white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 480.87 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.82 g cm −3 (80 ° C) |
|||||||||||||||
| Melting point |
43-45 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| Refractive index |
1.4398 (70 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cetyl palmitate , also known as cetyl ester wax , palmitic acid cetyl ester and whale rate substitute , is the ester of saturated C 16 carboxylic acid ( palmitic acid ) and saturated C 16 alcohol ( cetyl alcohol ). It is used in the cosmetics industry as a moisturizing thickener for ointments and creams, earlier in the mineral oil industry as a lubricant additive.
History and occurrence
Cetyl palmitate, an animal wax, used to be extracted from the whale rat , which occurs in amounts of up to 5 tons in the head cavities of the sperm whale . Cetyl palmitate is the main component of whale rat. Since the whaling - moratorium in 1985 synthetic cetyl palmitate is used.
Extraction and presentation
The whale rat, which used to be obtained from natural sources, was further processed directly; today's synthetic cetyl palmitate is obtained by esterifying natural saturated and unsaturated fatty acids and saturated fatty alcohols . The fatty acids required are obtained by fractional distillation of the corresponding saturated fatty acids; the fatty alcohols, for example, from natural fats by hydrolysis and subsequent hydrogenation . The commercially available "cetyl palmitate" is a mixture of different esters with an average molar mass of around 500 g · mol −1 .
properties
Cetyl palmitate is used in creams and ointments up to a level of 3%. The cream / ointment produced in this way should be smoother with a comparable viscosity than when using, for example, cetyl alcohol or beeswax. The creams have moisturizing and smoothing properties.
use
Cetyl palmitate is used as a high-quality consistency agent for ointments and is a component of the cooling ointment (Unguentum leniens) according to the German Pharmacopoeia.
Individual evidence
- ↑ a b c d e f Data sheet cetyl palmitate (PDF) from Merck , accessed on January 19, 2011.
- ↑ Safety data sheet (Caelo) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-278.
Web links
- Recipe information: Creams (lipophilic) of the new recipe form (PDF file; 61 kB)