Chlormadinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
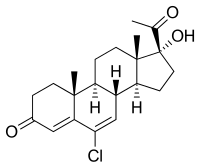
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Chlormadinone | ||||||||||||||||||
| other names |
6-chloro-17 α- hydroxy-17 β -pregna-4,6-diene-3,20-dione |
||||||||||||||||||
| Molecular formula | C 21 H 27 ClO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 362.89 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chlormadinone is a synthetically produced sex hormone ( progestin ). It is used to treat hormonal disorders in women, for hormone replacement therapy during menopause and in combination with ethinylestradiol for hormonal contraception ( birth control pills ).
Mode of action
Chlormadinone has an anti- estrogenic effect. It reduces the mobility of the fallopian tubes and makes the cervical mucus thicker. The egg transport is disrupted and the sperm cells from entering the uterus are prevented. In higher doses, chlormadinone inhibits the release of gonadotropic hormones from the pituitary gland . The gonadotropic hormones LH and FSH control the activity of the ovaries (e.g. ovulation) and transformation processes in the uterine lining .
Chlormadinone also has a partial antiandrogenic effect, which is based on the substance's ability to displace androgens from their receptors. This is important for the therapy of androgen-dependent diseases such as hirsutism , androgenetic hair loss and acne seborrhoica .
A pregnancy-sustaining effect, as with other synthetic gestagens, could not be proven for chlormadinone.
application areas
Chlormadinone is used as chlormadinone acetate in drugs. It is indicated in hormone replacement therapy for menopausal symptoms, for the treatment of growths of the uterine lining , irregular cycles and menstrual cramps (e.g. oligomenorrhea , polymenorrhea , hypermenorrhea , dysmenorrhea , secondary amenorrhea ) and painful feelings of tightness in the chest ( mastodynia ) . In combination with ethinylestradiol , it is used for hormonal contraception.
Patients suffering from androgenetic diseases (such as acne, see section on mode of action ) can benefit therapeutically from the anti-androgenic effect of chlormadinone acetate.
Trade names
Balanca (D, A), Belara (D, A, CH), Bellissima (D), Chantal (D), Chariva (D), Esticia (D), Enriqa (lactose-free) (D), Eufem (D), Lilia (D), Madinette (D), Minette (D) Mona Hexal (D), Neo Eunomin (D), Pink Luna (D)
Chlormadinone 2mg fem (D)
Web links
- Combined contraception with new progestin: Belara ( Memento from January 15, 2009 in the Internet Archive )
Individual evidence
- ↑ a b data sheet Chlormadinone acetate at Sigma-Aldrich , accessed on August 17, 2019 ( PDF ).
- ↑ Entry on chlormadinone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ JC Frölich, W. Kirch (ed.): Practical medicine therapy , Springer-Verlag, 2003, ISBN 3-540-01025-4 , p. 435.
- ↑ Treatment of acne vulgaris . ( Memento of November 27, 2015 in the Internet Archive ) In: arznei-telegram No. 1 (1997).
- ^ Druckmann R: Profile of the progesterone derivative chlormadinone acetate - pharmocodynamic properties and therapeutic applications. . In: Contraception . 79, 2009, pp. 272-281. PMID 19272496 .