Cobalt (II) sulfate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
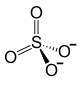
|
|||||||||||||
| General | |||||||||||||
| Surname | Cobalt (II) sulfate | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula |
|
||||||||||||
| Brief description |
carmine crystals (heptahydrate) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
|
||||||||||||
| Melting point |
Decomposes at 735 ° C |
||||||||||||
| solubility |
good in water (383 g l −1 at 25 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic, toxic for reproduction ( CMR ) |
||||||||||||
| MAK |
repealed because it is carcinogenic |
||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
In the anhydrous state, cobalt (II) sulfate is a violet-tinged red ("pink"), hygroscopic salt of sulfuric acid . The poisonous, carcinogenic powder absorbs water of hydration in moist air and forms the carmine-red cobalt (II) sulfate heptahydrate. Depending on the temperature, the hexahydrate and the monohydrate also occur.
Occurrence
Cobalt (II) sulfate rarely occurs in the form of some crystal hydrate minerals (Co [SO 4 ] · xH 2 O) along the oxidation zones of primary cobalt minerals (such as skutterudite or cobaltite ). These are: bieberite (heptahydrate), moorhouseit (hexahydrate), aplowite (tetrahydrate) and cobalt kieserite (monohydrate).
The recycling of lithium-ion batteries is being researched. This is becoming more important because such batteries are playing an increasing role in electromobility and cobalt as a raw material plays a prominent role in it.
Extraction and presentation
Cobalt (II) sulfate is formed when sulfuric acid acts on elemental cobalt or on cobalt oxides such as cobalt (II) oxide .
properties
Anhydrous cobalt (II) sulfate is polymorphic and crystallizes as an orthorhombic A-form or monoclinic B-form. The hepta- and monohydrate form monoclinic crystal structures. The heptahydrate splits off water of crystallization at 41.5 ° C and forms the also red hexahydrate. This in turn is converted into pink monohydrate by splitting off crystal water at 71 ° C.
use
Cobalt (II) sulphate is used for the production of pigments , glazes , in porcelain painting , for toning papers (photography), in baths for cobalt electroplating and for trace element supplementation in aquariums and the like. a. used.
In addition, the salt was added to beer in the 1960s to increase the stability of the foam. As a result, a number of cases of cobalt-induced cardiomyopathy (cobalt cardiomyopathy) have occurred in Canada and the United States . 49 patients were registered in Quebec and 64 in Omaha. Symptoms included stomach pain, weight loss, nausea, shortness of breath, and coughing, among others. The mortality rate was around 40%. Autopsies revealed severe damage to the heart muscle and liver . All patients were heavy beer drinkers (1.5 to 3 liters / day). They preferred to consume varieties from local breweries that had started adding cobalt (II) sulfate to beer as a foam stabilizer about a month earlier . The limit values for cobalt in food had not been exceeded. The incidence of the disease came to a standstill immediately after the breweries discontinued the cobalt (II) sulfate admixtures.
Individual evidence
- ↑ a b c d e f g h Entry on cobalt (II) sulphate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b SLAC: PHYSICAL CONSTANTS OF INORGANIC COMPOUNDS (PDF; 391 kB)
- ↑ Entry on Cobalt sulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on December 6, 2019.
- ↑ Data sheet cobalt (II) sulfate (PDF) from Merck , accessed on January 19, 2011.
- ↑ Jingu Kang, Gamini Senanayake, Jeongsoo Sohn, Shun Myung Shin: Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. In: Hydrometallurgy . Volume 100, number 3-4, 2010, pp. 168-171. DOI: 10.1016 / j.hydromet.2009.10.010 .
- ^ JD Dunitz, P. Pauling: Polymorphism in Anhydrous Cobalt Sulphate . In: Acta Crystallographica . tape 18 , no. 4 , 1965, pp. 737-740 , doi : 10.1107 / S0365110X65001688 .
- ↑ a b C. Thomas: Special Pathology. Schattauer Verlag, 1996, ISBN 3-7945-2110-2 , p. 179 ( limited preview in the Google book search).
- ↑ Expert Group on Vitamins and Minerals. 2002.
- ^ Cardiology: When Beer Brought the Blues. In: New York Times January 10, 1967 issue.



