Cyclohexanethiol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
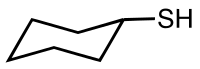
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclohexanethiol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 S | ||||||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 116.23 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.95 g cm −3 |
||||||||||||||||||
| Melting point |
−118 ° C |
||||||||||||||||||
| boiling point |
158-160 ° C |
||||||||||||||||||
| Vapor pressure |
5 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.493 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cyclohexanethiol is a chemical compound from the group of thiols .
Occurrence
Cyclohexanethiol occurs as a decomposition product of cyclohexylthiophthalimide (used as a polymerization inhibitor in rubber production).
Extraction and presentation
Cyclohexanethiol can be obtained by reacting cyclohexene (for example by Markovnikov addition) or cyclohexanol or a cyclohexyl halide with hydrogen sulfide .
The compound can also be prepared by saponification of cyclohexyldithiochlorocarbonic acid ester with alcoholic potassium hydroxide solution .
Synthesis by pyrolysis of 1,2-epithiocyclohexane at 210 ° C., by hydrolysis of cyclohexylthiol acetate and other processes is also possible.
properties
Cyclohexanethiol is a colorless liquid with an unpleasant odor that is practically insoluble in water. Of the 24 possible conformations of the compound, the equatorial chair formation is the most stable.
use
Cyclohexanethiol is used as an intermediate in the manufacture of other chemical compounds (such as cyclohexylthiophthalimide).
safety instructions
The vapors of cyclohexanethiol can form an explosive mixture with air ( flash point 39 ° C, ignition temperature 253 ° C).
Individual evidence
- ↑ a b c d e f g h i j Entry on cyclohexanethiol in the GESTIS substance database of the IFA , accessed on January 27, 2019(JavaScript required) .
- ↑ CYCLOHEXANETHIOL: ICSC 0032 - CYCLOHEXANETHIOL , accessed January 27, 2019
- ↑ a b c Werner Baumann, Monika Ismeier: Rubber and rubber data and facts on environmental protection . Springer-Verlag, 2013, ISBN 978-3-642-58916-4 , pp. 1038 ( limited preview in Google Book search).
- ↑ Data sheet cyclohexanethiol, 97% from Sigma-Aldrich , accessed on January 27, 2019 ( PDF ).
- ↑ Eula Bingham, Barbara Cohrssen: Patty's Toxicology, 6 Volume Set . John Wiley & Sons, 2012, ISBN 0-470-41081-7 , pp. 1054 ( limited preview in Google Book search).
- ↑ a b Mohamed A. Metwally, Bakr F. Abdel-Wahab: ChemInform Abstract: Utility of Cyclohexanethiols in Organic Synthesis. In: ChemInform. 41, 2010, S. no, doi : 10.1002 / chin.201027249 .
- ↑ Entry on cyclohexanethiol in the Hazardous Substances Data Bank , accessed on January 27, 2019.
- ^ A b John S. Dick, Charles P. Rader: Raw Materials Supply Chain for Rubber Products Overview of the Global Use of Raw Materials, Polymers, Compounding Ingredients, and Chemical Intermediates . Carl Hanser Verlag GmbH Co KG, 2014, ISBN 978-1-56990-538-8 , pp. 419 ( limited preview in Google Book search).
- ^ Eugen Müller: Houben-Weyl Methods of Organic Chemistry Vol. IX, 4th Edition Sulfur, Selenium, Tellurium Compounds . Georg Thieme Verlag, 2014, ISBN 3-13-180544-7 , p. 18 ( limited preview in Google Book search).
- ^ DW Scott, GA Crowder: Cyclohexanethiol and 2,4-Dimethyl-3-thiapentane: Molecular Vibrations, Conformational Analyzes, and Chemical Thermodynamic Properties. In: The Journal of Chemical Physics. 46, 1967, p. 1054, doi : 10.1063 / 1.1840768 .
- ↑ Sang-Woo Joo, Hoeil Chung and a .: Conformational changes of cyclohexanethiol adsorbed on gold surfaces. In: Surface Science. 601, 2007, p. 3196, doi : 10.1016 / j.susc.2007.05.020 .


