Cyclopropylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
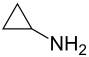
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclopropylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 7 N | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 57.10 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.82 g cm −3 |
||||||||||||||||||
| Melting point |
−10 ° C |
||||||||||||||||||
| boiling point |
49-50 ° C |
||||||||||||||||||
| Vapor pressure |
322 hPa (20 ° C) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| Refractive index |
1.4210 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
45.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cyclopropylamine ( cyclopropanamine according to IUPAC ) is a primary amine . The compound is liquid at room temperature and volatile because of its relatively low boiling point . Like many amines, cyclopropylamine has an ammonia to fishy odor.
properties
Chemical properties
Cyclopropylamine is an organic base and therefore forms with various acids such as. B. the hydrochloric acid salts of the type C 3 H 5 -NH 3 + Cl - . The reactivity is strongly influenced by the nucleophilicity of nitrogen . It reacts, for example, with carboxylic acid halides to form amides and with aldehydes / ketones to form imines or, through reductive amination, to form corresponding secondary or tertiary amines.
The bond angles between carbon - atoms in the cyclopropyl ring is approximately 60 °, the bond distance of 151 pm is a single bond relatively short. Hence the ring is stretched and planar. These spatial properties are used, among other things, in pharmaceutical research for various purposes, for example as a “suitable” structural element for certain receptors .
use
Cyclopropylamine is used, among other things, in pharmaceutical chemistry as a starting material for the production of new active ingredients .
Biological importance
Cyclopropylamine as such has no biological significance for humans, animals or plants.
safety instructions
The connection is corrosive , so you should protect yourself with gloves. Storage and handling in well-ventilated rooms. The LD 50 value is 445 mg / kg (rat, oral).
Individual evidence
- ↑ a b c Data sheet cyclopropylamine (PDF) from Merck , accessed on January 18, 2011.
- ↑ a b c d e f Datasheet Cyclopropylamine from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-132.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.


