p -Cymol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
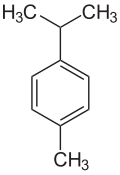
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | p -Cymol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 14 | |||||||||||||||
| Brief description |
colorless liquid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 134.22 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.86 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−68 ° C |
|||||||||||||||
| boiling point |
177 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
practically insoluble in water (34 mg l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4907 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
p -Cymol ( para -Cymol ), also known as dolcymen or camphogen , is an aromatic hydrocarbon , more precisely an alkylbenzene , and belongs to the monocyclic monoterpenes . It is a colorless, flammable liquid with a characteristic odor. Together with its two isomers, it belongs to the cymene group and also to the C 4 benzenes .
Biological importance
p -Cymol has no proven biological role, but it is found in many plants. The three most important plants are Chenopodium ambrosioides (Epazote) (730 to 8000 ppm in the plant), Peumus boldus (Boldo) (6000 to 7500 ppm in the leaves) and Satureja hortensis (savory) (300 to 6000 ppm in the plant) .
use
p -Cymol is a common ligand for ruthenium complexes. The starting compound is [(η 6 -Cymol) RuCl 2 ] 2 . This half sandwich complex is produced by the reaction of ruthenium (III) chloride with the terpene α-Phellandren . The osmium complex is also known.
safety instructions
The flash point of the liquid is 47 ° C, the ignition temperature is 435 ° C. In air volume proportions of 0.7 to 5.6% it forms explosive mixtures. A MAK value has not been established for p -cyymol.
When inhaled, p- cymene causes dizziness, drowsiness and vomiting. It degreases the skin and reddens the skin and eyes. If swallowed, the liquid causes diarrhea, headache, nausea, loss of consciousness, vomiting and drowsiness.
Individual evidence
- ↑ Entry on P-CYMENE in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d e f g h i j k l m n o p q Entry on 4-isopropyltoluene in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ RT O'Connor, LA Goldblatt: Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons , in: Anal. Chem. , 1954 , 26 , pp. 1726-1737 ( doi: 10.1021 / ac60095a014 ).
- ↑ Dr. Duke's Phytochemical and Ethnobotanical Databases: P-Cymol , accessed February 5, 2018.
- ↑ MA Bennett, T.-N. Huang, TW Matheson, AK Smith, Steven Ittel, William Nickerson: (η 6 -Hexamethylbenzene) Ruthenium Complexes , in: Inorganic Syntheses , 1982 , 21 , pp. 74-78 ( doi: 10.1002 / 9780470132524.ch16 ).


