Dibenzepine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
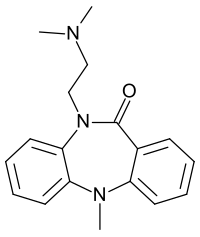
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Dibenzepine | |||||||||
| other names | ||||||||||
| Molecular formula | C 18 H 21 N 3 O | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| Drug class | ||||||||||
| Mechanism of action |
non-selective monoamine reuptake inhibitor |
|||||||||
| properties | ||||||||||
| Molar mass | 295.38 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
116-117 ° C |
|||||||||
| boiling point |
185 ° C (1.3 Pa ) |
|||||||||
| pK s value |
8.25 (monohydrochloride) |
|||||||||
| solubility |
soluble in ethanol , chloroform , water (monohydrochloride) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dibenzepine (manufacturer: Novartis ) is a tricyclic antidepressant that came onto the German market in 1965 . Its chemical structure is related to the benzodiazepines and some neuroleptics ( clozapine , olanzapine , quetiapine , zotepine ), but without sharing their pharmacological properties. Dibenzepine has a relatively short half-life of approx. 9 hours with a plasma binding of 80%. It has a sedative , antidepressant and anxiolytic effect . It has the typical side effects of the tricyclic antidepressants (see there ).
Therapeutically, doses of 240 to 720 mg are used daily. In contrast to other tricyclic antidepressants, toxic plasma levels of dibenzepine are significantly higher (over 3000 ng / ml).
Use in pregnancy
There is clear evidence of risks to the human fetus, but the therapeutic benefits to the mother may outweigh the risks. Dibenzepine should only be used during pregnancy if absolutely necessary.
Effects on ability to drive and use machines
Dibenzepine can reduce responsiveness. This must be taken into account in relation to the ability to drive and use machines.
Trade names
Noveril TR (CH), Noveril retard (A)
Individual evidence
- ↑ a b c Entry on dibenzepine. In: Römpp Online . Georg Thieme Verlag, accessed on July 7, 2014.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, ISBN 978-0-911910-00-1 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on dibenzepine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Psychiatric Drugs Timeline .
- ↑ a b c d Specialist information Noveril ® TR, Swiss Medicines Compendium, as of June 2010.
- ↑ Laborlexikon.de .
- ↑ AGES-PharmMed, as of June 2010.