Dihydropyrimidine dehydrogenase
| Dihydropyrimidine dehydrogenase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 1022 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Cofactor | FAD, FMN, ( 4Fe-4S ) | |
| Identifier | ||
| Gene name | DPYD | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.3.1.2 , oxidoreductase | |
| Response type | Redox reaction | |
| Substrate | 5,6-dihydrouracil + NADP + | |
| Products | Uracil + NADPH + H + | |
| Occurrence | ||
| Parent taxon | Eukaryotes , bacteria | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 1806 | 99586 |
| Ensemble | ENSG00000188641 | ENSMUSG00000033308 |
| UniProt | Q12882 | Q8CHR6 |
| Refseq (mRNA) | NM_000110 | NM_170778 |
| Refseq (protein) | NP_000101 | NP_740748 |
| Gene locus | Chr 1: 97.08 - 97.92 Mb | Chr 3: 118.56 - 119.43 Mb |
| PubMed search | 1806 |
99586
|
The dihydropyrimidine dehydrogenase (DPD) is an enzyme from the group of oxidoreductases , comprising a step in the degradation of endogenous pyrimidines such as uracil and thymine catalyzed. In addition, it breaks down various cytostatics such as 5-fluorouracil (5-FU) and capecitabine . A hereditary disease, the DPD deficiency (E79 according to ICD-10), means that these cytostatics accumulate in the body during cancer therapy and can cause life-threatening poisoning. Blood tests for this genetic disorder are available commercially.
Catalyzed reactions
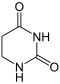
Uracil is hydrogenated to dihydrouracil .
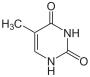
Thymine is hydrogenated to 5,6-dihydrothymine .
The catalyzed reaction is reversible and rate-determining, that is, an equilibrium is established depending on the availability of the starting and end products. The enzyme is found in all eukaryotes, including the slime mold Dictyostelium , and in many bacteria. A close relative in plants is dihydroorotate reductase .
Importance and inhibition
In about 5% of the patients who received 5-fluorouracil as a cytostatic agent, severe symptoms of intoxication including cardiac arrhythmia occurred. Occasional deaths have been reported. The reason for these reactions is a genetic, reduced activity of the dihydropyrimidine dehydrogenase.
The enzyme is inhibited by various substances, among others
- Bromovinyluracil , a breakdown product of the virostat brivudine (this is why the simultaneous administration of 5-FU or its precursors and brivudine can lead to dangerous interactions),
- Uracil ,
- Eniluracil .
Individual evidence
- ↑ S. Maurer, J. Thödtmann: The mammary carcinoma: diagnosis and therapy. Govi-Verlag, Eschborn, 2003, ISBN 978-3-7741-0996-4 .
- ↑ Uniprot entry
- ↑ H. Müller: Genetic tests: Answers to questions from medical practice. Karger Publishers, 2005, ISBN 978-3-8055-7820-2 .