Capecitabine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
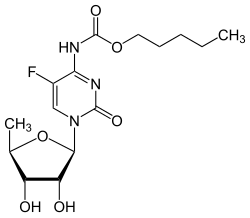
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Capecitabine | |||||||||||||||||||||
| other names |
N 4 -pentyloxycarbonyl-5'-deoxy-5-fluorocytidine |
|||||||||||||||||||||
| Molecular formula | C 15 H 22 FN 3 O 6 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 359.35 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
110-121 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Capecitabine (trade name Xeloda ® , manufactured by Roche ) is a cytostatic -acting drug . Capecitabine is a precursor ( prodrug ) of 5-fluorouracil and is converted into the active substance in the tumor. The substance is active orally and is used for the therapy of metastatic colon cancer , metastatic or locally advanced breast cancer and for the palliative therapy of gastric cancer .
Pharmacokinetics
Capecitabine is a prodrug that is rapidly absorbed from the gastrointestinal tract with a maximum plasma concentration after 90 minutes. The plasma protein binding is less than 60%. Capecitabine is in the liver hydrolyzed to 5'-deoxy-5-fluorocytidine, which in cells to 5'-deoxy-5-fluorouridine ( doxifluridine is converted), and further to the active ingredient 5-fluorouracil (5-FU).
The effectiveness of capecitabine is therefore comparable to that of 5-FU. The conversion to 5-FU takes place by the enzyme thymidine phosphorylase, which occurs in particularly high concentrations in tumor tissue. Due to the targeted alignment of the mechanism of action on the tumor cells, the patients tolerate capecitabine better and have to be treated significantly less often because of severe side effects.
The breakdown of capecitabine and 5-FU takes place via the enzyme dihydropyrimidine dehydrogenase .
Side effects
Like most cytotoxic drugs, capecitabine can cause a variety of mild, severe, and even fatal side effects. Muscle or limb pain, exhaustion, changes in the blood count, infections, disorders of the nervous system, sore throat, symptoms of the gastrointestinal tract, liver and kidney damage, and circulatory disorders in the heart, including heart attacks, are particularly common. In particular, nausea, vomiting and, above all, stomatitis are less common than with 5-FU . Hair loss is also observed much less frequently. However, severe diarrhea can occur even with capecitabine. Hand-foot syndrome occurs much more frequently with oral medication . Symptoms range from numbness, tingling, and abnormal sensations to severe pain in the palms of the hands and soles of the feet. There may also be ulcers and blisters on the hands or feet. Those affected can benefit from regular cold water baths for hands and feet or 10% uridine-containing creams. The loss of fingerprints cannot be ruled out either.
Serious skin reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) can very rarely occur during therapy with capecitabine . If there are any signs, the treatment must be stopped immediately and permanently. The manufacturer informed about this risk on December 17, 2013 in a red-hand letter .
application
Capecitabine is taken twice a day, morning and evening, within 30 minutes of a meal. Depending on the dosage, the patient has to swallow three to nine tablets at a time. In the event of severe side effects, therapy must be interrupted or the dose reduced.
Web links
- Xeloda on the website of the European Medicines Agency
- Pharmaceutical newspaper: New drugs: Capecitabine
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Capecitabine
Individual evidence
- ↑ Entry on capecitabine. In: Römpp Online . Georg Thieme Verlag, accessed on October 1, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ S. Maurer, J. Thödtmann: The mammary carcinoma: diagnosis and therapy. Govi-Verlag, Eschborn, 2003, ISBN 978-3-7741-0996-4 .
- ↑ Roche AG: specialized information Xeloda , Stand September 14, 2016
- ↑ Hendrik-Tobias Arkenau: Therapy Management: Capecitabine and the Hand-Foot Syndrome . 2006. Retrieved April 13, 2009.
- ↑ Unexpected side effect: entry ban . Retrieved September 8, 2019.
- ^ Wong M, Choo SP, Tan EH: Travel warning with capecitabine . In: Ann. Oncol. . 20, No. 7, July 2009, p. 1281. doi : 10.1093 / annonc / mdp278 . PMID 19470576 .
- ↑ Drug Commission of the German Medical Association: Drug Safety Mail 2013-68 - Rote-Hand-Brief on Xeloda® (capecitabine): Risk of severe skin reactions . 17th December 2013.