Xanthates
Xanthogenate is an outdated term for salts of O - alkyl esters of dithiocarbonic acid and for the O , S - dialkyl esters (xanthic acid esters, alkyl xanthates) accessible from them . They are carbonic acid derivatives in which two oxygen atoms have been replaced by sulfur. They contain R 1 –O – CSS - or R 1 –O – CSS – R 2 as a functional group .

presentation
Salt-like xanthates can be prepared from alcoholates by reaction with carbon disulfide:
The esters are obtained from the salts by alkylation:
Reactions
The pyrolysis of alkylated xanthates produces alkenes in the Chugayev reaction . Since the reaction mechanism - comparable to that of a decarboxylation - can be described with a cyclic transition state, the Chugayev reaction proceeds as a stereochemically unambiguous syn - elimination .
By the Barton-McCombie reaction is converted an alcohol in the xanthate to him subsequently with tributyl tin hydride or hexamethyldisilazane radical to the alkane to defunctionalization.
Applications
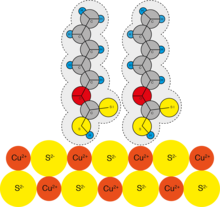
Xanthates (such as potassium O -ethyldithiocarbonate and sodium O -ethyldithiocarbonate ) are used as anion-active collectors in the flotation of lead and copper ores.
Fibers based on cellulose can be used as a lining material in producing high quality textiles.
The production of fibers or foils (" cellophane ") based on cellulose can be done using the xanthate process. This reaction procedure was patented in 1892 by Charles Frederick Cross and Edward John Bevan , the first film production by reprecipitation of the cellulose phase in 1898 by Charles Henry Stearn. The pulp is first treated with caustic soda for a few hours ( mercerization ). With the addition of carbon disulfide, the xanthate is formed within two to three hours; only some of the hydroxyl groups of the glucose units are esterified. The mass turns brown due to the formation of the by-product sodium trithiocarbonate. This mass is then diluted by adding more sodium hydroxide solution. The result is a colloidal , highly viscous brown solution ("viscose solution"), which gave the end product its vague name: "viscose". Spinning the viscous solution by pressing the threads into a sulfuric acid spinning bath then provides again cellulose, also known as "viscose silk" or rayon is known (or viscose ).
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 652, ISBN 3-342-00280-8 .
- ^ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, pp. 158–159, ISBN 3-211-81060-9 .
- ↑ tachemie.uni-leipzig.de: Flotation ( Memento from November 2, 2013 in the Internet Archive ), accessed on May 11, 2009.
- ↑ British patent 8700/1892, also published as DRP: Patent DE70999 : Production of a water-soluble derivative of cellulose, called "Viscoid". Published September 5, 1893 , Inventors: Charles Frederick Cross, Edward John Bevan, Clayton Beadle.
- ↑ Patent GB189801022 : Improvements in the Manufacture and Production of a material in film, sheet, or web form. Published December 3, 1898 , inventor: Charles Henry Stearn.
- ^ Bertram Philipp, Peter Stevens: Grundzüge der Industrielle Chemie , VCH Verlagsgesellschaft mbH, 1987, p. 304, ISBN 3-527-25991-0 .