Ductal carcinoma in situ
| Classification according to ICD-10 | |
|---|---|
| D05.1 | Carcinoma in situ of the milk ducts |
| ICD-10 online (WHO version 2019) | |
A ductal carcinoma in situ (English ductal carcinoma in situ - DCIS) is an abnormal proliferation of neoplastic cells in the milk ducts (Ductuli) of the female breast . This also includes Paget's carcinoma (English: paget disease of the nipple , after the English surgeon James Paget ), in which only the skin of the nipple is infiltrated.
DCIS is about degenerate cells, which, however, have not yet broken through the boundary of the milk duct ( basement membrane ) ("in place", in situ ). Such an early carcinoma is always curable because it does not spread daughter cells. When the tumor (tissue knot) breaks the barrier into the neighborhood, the growth is called invasive . Large DCIS foci (> 2 cm) often contain invasive areas that can only be found with meticulous histological preparation. DCIS make up about 15% of all breast cancers .
Not all of these so-called in-situ breast carcinomas grow in the course of the disease ( progress ), but some can develop into an invasive carcinoma. The DCIS is therefore considered a precancerous stage ( precancerous disease ). It is estimated that about 50% of in situ cancers will develop into invasive cancers over a period of 10–20 years. Since DCIS, unlike invasive breast cancer, is almost 100% curable if it is completely removed, the detection of this precancerous condition is a declared aim of mammography screening. The changes should be removed in good time and completely before an invasive breast cancer develops. The treatment is therefore on the one hand a preventive measure to avoid breast cancer , on the other hand the surgical therapy takes place because a microinvasion (a microscopic invasion) cannot be excluded with mammography alone.
Symptoms
In the vast majority of cases, DCIS occurs without symptoms and is usually only discovered during a mammogram. Rare symptoms include chest pain, a palpable tumor, or bloody secretion from the nipple.
Diagnosis
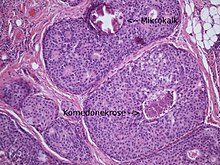
Ductal in situ carcinomas are sometimes palpable (so-called “tumor-forming DCIS”), in most cases they can only be detected with the help of mammography. So-called micro - calcifications are typical , which can be punctured by the radiologist with the help of a vacuum biopsy in order to obtain small ( minimally invasive ) samples. An operation to confirm the diagnosis is therefore only rarely necessary (e.g. if it is located directly on the chest wall or under the skin). The histological examination is then carried out by a pathologist . A ductal in situ carcinoma can only be diagnosed with technical aids ( mammography , histology ) because it is not visible to the naked eye.
According to the WHO classification of tumors, the DCIS is divided into three grades based on its morphology : low grade of core malignancy, intermediate grade and high grade of core malignancy. They give a rough indication of the aggressiveness of the tumor and are determined with the help of the core diameter of the tumor cells.
The higher the core grade, the more aggressive the tumor is. In DCIS with a medium or high degree of nuclear malignancy, so-called comedo necrosis often occur, which can calcify and thus become visible in the mammography. The comedone necrosis is interpreted as a sign of rapid cell growth.
If there are several different degrees in a tumor, the highest degree is always given in the histological diagnosis.
The encryption according to the TNM classification is Tis (Carcinoma i n s itu) with the addition DCIS .
therapy
The therapy of the DCIS consists of an operative removal in the healthy, i. H. a complete excision with a sufficient safety margin to healthy tissue (S3 guidelines of the German Cancer Society : 5 mm. European guidelines: 10 mm safety margin). Breast-conserving surgery is usually possible for small in-situ carcinomas (<4 cm). In the case of very large lesions or an unfavorable tumor-to-breast ratio, a mastectomy (often with prompt plastic-surgical reconstruction of the affected breast) is performed.
Chemotherapy is not necessary. A general recommendation for anti-hormonal treatment of DCIS (e.g. with tamoxifen ) no longer exists in the S3 guidelines . A benefit has only been scientifically proven for DCIS with a high degree of core malignancy, young patients (<50 years) and affected resection margins, whereby the benefit is independent of the determined hormone receptor status.
forecast
There are currently no reliable prognostic factors for the DCIS. This means that even if all the characteristics of the disease (e.g. core degree and size) are known, no prediction can be made about the probable course in an individual case. The most important factors for the development of a relapse are the age of the patient (<45 years) and any residual tumor in the breast after the operation. Avoiding recurrence is important in that 50% of all DCIS recurrences occur as invasive breast cancer.
If it is completely removed with sufficient safety margins, the prognosis of the DCIS is extremely good.
Van Nuys Forecast Index
The Van Nuys Forecast Index (VNPI) was developed in 1996 at the University of South California. The basis was the analysis of data from 706 patients with DCIS who had undergone breast-conserving surgery. The VNPI includes the grade of nuclear malignancy, the size of the tumor, the age of the patient and the distance of the DCIS from the resection margin.
Despite its popularity, the Van Nuys prognosis index is viewed critically today, especially because only retrospective data are available and not the results of a prospective randomized study. For this reason, its use for prognostic assessment is not recommended in the current S3 guidelines, among other things because a removal with a safety margin of at least 5 mm should always be carried out during an operation in order to prevent a relapse.
Problems and current scientific status
From a scientific point of view, the DCIS is still a "new" disease, i. H. it has only been known for a few decades. The frequent occurrence (approx. 15% of all malignant breast diseases) nowadays is mainly due to mammography screening. This has the effect that DCIS is discovered much earlier as a precancerous stage, namely before an invasive growth or a palpable tumor is present.
It is known that the risk of developing breast cancer is 8 to 11 times higher with ductal in situ carcinoma than with a healthy breast. However, not every DCIS degenerates into an invasive carcinoma. Since, by definition, in-situ carcinoma can not metastasize and is therefore not life-threatening, removal is used solely to prevent breast cancer.
The current scientific status is reflected in the medical S3 guidelines ( medical guidelines with the highest quality level) of the German Cancer Society, which were developed in cooperation with gynecologists , radiologists , pathologists , oncologists and patient representatives.
See also
literature
- Pathology and Genetics of Tumors of the Breast and Female Genital Organs (Who / IARC Classification of Tumors). 2003, ISBN 92-832-2412-4 .
Web links
- Breast disorders: carcinoma in situ, Van-Nuys prognostic index
- S3 guideline for breast cancer of the German Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB)
- European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis (PDF; 2.4 MB)
- German Cancer Society guidelines for affected patients (PDF; 578 kB)
Individual evidence
- ^ HJ Burstein et al .: Ductal Carcinoma in situ of the Breast . In: N Engl J Med , 2004, 350, pp. 1430-1441, doi: 10.1056 / NEJMra031301
- ^ A. Lebeau: [Prognostic factors in ductal carcinoma in situ]. In: The Pathologist. Volume 27, Number 5, September 2006, pp. 326-336, doi : 10.1007 / s00292-006-0853-y , PMID 16896676 .
- ^ Robert J. Rosser: Consensus conference on the classification of ductal carcinoma in Situ. In: Cancer. 82, 1998, pp. 2293-2294, doi : 10.1002 /% 28SICI% 291097-0142% 2819980601% 2982: 11% 3C2293 :: AID-CNCR29% 3E3.0.CO; 2-O .
- ↑ S3 guideline for breast cancer of the Dt. Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB) p. 232
- ↑ European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis (PDF; 2.4 MB) p. 331
- ↑ J. Houghton, WD George, et al .: Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomized controlled trial. In: Lancet. Volume 362, Number 9378, July 2003, pp. 95-102, PMID 12867108 .
- ↑ S3 guideline for breast cancer of the Dt. Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB) pp. 33–34
- ↑ N. Bijker et al .: Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organization for Research and Treatment of Cancer randomized phase III trial 10853 - a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group . In: J Clin Oncol . , 2006 Jul 20, 24 (21), pp. 3381-3387, PMID 16801628
- ↑ N. Bijker et al .: Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853 . In: J Clin Oncol. , 2001 Apr 15, 19 (8), pp. 2263-2271, PMID 11304780
- ↑ Kai C. Chan, W. Fiona Knox et al. a .: Extent of excision margin width required in breast conserving surgery for ductal carcinoma in situ. In: Cancer. 91, 2001, pp. 9-16, doi : 10.1002 / 1097-0142% 2820010101% 2991: 1% 3C9 :: AID-CNCR2% 3E3.0.CO; 2-E .
- ^ C. Vargas, et al .: Factors associated with local recurrence and cause-specific survival in patients with ductal carcinoma in situ of the breast treated with breast-conserving therapy or mastectomy. In: International journal of radiation oncology, biology, physics. Volume 63, Number 5, December 2005, pp. 1514-1521, doi : 10.1016 / j.ijrobp.2005.04.045 , PMID 16005576 .
- ↑ W. Böcker: Preneoplesia of the Breast - An New Conceptual Approach to Proliferative Breast Disease . Elsevier, 2006, ISBN 0-7020-2892-4 , p. 513.
- ^ MJ Silverstein et al .: A prognostic index for ductal carcinoma in situ of the breast . Cancer . 1996 Jun 1, 77 (11), pp. 2267-2274, PMID 8635094 .
- ↑ S3 guideline for breast cancer of the Dt. Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB) p. 180
- ↑ S3 guideline for breast cancer of the Dt. Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB) pp. 28–29, p. 232
- ^ WHO Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs (Who / IARC 2003). ISBN 92-832-2412-4 , p. 67.
- ↑ S3 guideline for breast cancer of the Dt. Cancer Society ( Memento from January 18, 2012 in the Internet Archive ) (PDF; 1.67 MB)