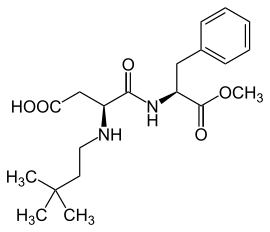
Neotame
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Neotame | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 30 N 2 O 5 | ||||||||||||||||||
| Brief description |
colorless crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 378.46 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
80-83 ° C or 80.9-83.4 ° C |
||||||||||||||||||
| solubility |
soluble in water, ethanol and ethyl acetate |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Neotame is a sweetener that is synthesized from aspartame and 3,3-dimethylbutyraldehyde . Its sweetness is about 7,000–13,000 times stronger than that of sucrose . Furthermore, a taste-enhancing effect has been shown in various products. Neotame is more stable than aspartame when heated in the neutral pH range. In clinical studies, neotame shows no influence on blood sugar and insulin plasma concentrations and can therefore also be used for dietetic and diabetic food under certain circumstances and taking into account limited dosages. Patients suffering from phenylketonuria also have no negative effects to fear, as neotame is only used in very small quantities due to its high sweetening power. Accordingly, the amounts of phenylalanine released during its hydrolysis are minimal . This sweetener has been approved in Australia and New Zealand since 2001, as well as in the USA and Mexico . Neotame has also been approved in the EU since January 20, 2010 (by Directive 2009/163 / EC). The European Food Safety Authority (EFSA) has assessed the safety of neotame and issued an opinion. The EFSA comes to the conclusion that neotame is safe as a sweetener and flavor enhancer in food. The authority defines the acceptable daily intake ( ADI ) as 0 to 2 milligrams per kilogram of body weight and day. The sweetener has the E number E 961.
Individual evidence
- ↑ Entry on E 961: Neotame in the European database for food additives, accessed on June 28, 2020.
- ^ A b c d The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; Pp. 1118-1119, ISBN 978-0-911910-00-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of L-phenylalanine, N- (3,3-dimethylbutyl) -L-α-aspartyl-, 2-methyl ester in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on, is reproduced from a self-classification by the distributor 28th July 2019.
- ↑ EFSA : Scientific Opinion of the Panel on Food Additives, Flavors, Processing Aids and Materials in Contact with Food .