Emetine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
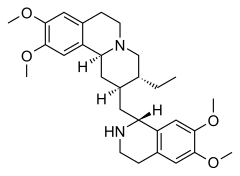
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Emetine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 29 H 40 N 2 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
inhibits acid sphingomyelinase |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 480.63 g mol −1 | |||||||||||||||||||||
| solubility |
Easily soluble in methanol , ethanol , acetone , ethyl acetate , diethyl ether , chloroform ; slightly soluble in water, petroleum ether ; moderately soluble in dilute ammonia; slightly soluble in potassium hydroxide and sodium hydroxide solution |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Emetine is a chemical compound from the group of alkaloids . It occurs naturally in the emetic root ( Carapichea ipecacuanha ) and has an anti- parasitic effect, promoting the expectoration of bronchial secretions and causing vomiting .
history
The name is derived from the ancient Greek ἔμεσις emesis , German 'vomiting' .
In 1817, François Magendie and Pierre Joseph Pelletier isolated the alkaloid emetine from the ipecacuanha root. In 1821 Magendie announced "Regulations for the preparation and use of emetine". Edward D. Vedder showed in 1912 that emetine can kill amoeba in vitro .
During the Second World War, emetine was made by the Indian company Cipla .
use
Emetine is one of the emetic active ingredients of the syrupus Ipecacuanhae ("emetic syrup " NRF , sugar syrup with Ipecacuanha tincture), which is used as an emetic for gastric emptying in the event of poisoning. Preparations made from ipecacuanha root were also used in the past for bronchial asthma and amoebic dysentery because of their alkaloid content (especially emetine and cephaelin ) . From the discovery of the amoebicidal effect of emetin, emetine salts were used for the parenteral treatment of diseases caused by Entamoeba histolytica or Leishmania tropica , in particular also of amoebic liver abscesses.
More recently, emetine is in their reaction to viruses ( Zika- and Ebola virus examined) and in cancer.
Mechanism of action
The expectorant effect arises from a reflex increase in bronchial secretion as a result of the local irritant effect of emetine in the gastrointestinal tract after oral administration. In higher doses, vomiting is also caused by local irritation.
The amebicidal effect of emetin comes about through the inhibition of protein synthesis and thus the mitosis of the vegetative forms of Entamoeba histolytika . Emetine does not work against permanent forms of the pathogen.
In vitro , an inhibition of the enzyme acid sphingomyelinase (ASM, EC 3.1.4.12 ) has been shown, so that emetine is classified as part of the FIASMA (“functional inhibitors of acid sphingomyelinase”).
unwanted effects
Side effects include muscle and nerve damage.
Salts of emetine
Emetine dihydrochloride, emetine dihydrochloride pentahydrate, emetine dihydrochloride heptahydrate, (+) - emetine dihydrochloride hydrate and emetine dihydrobromide-4-water are described as salts of emetin. Both emetine dihydrochloride pentahydrate and heptahydrate are each a white to slightly yellowish, crystalline powder and easily soluble in water and ethanol 96%.
literature
- A. Buzas, R. Cavier, F. Cossais, JP Finet, JP Jacquet, G. Lavielle, N. Platzer: Synthèse et propriétés amœbicides d'analogues de l'émétine. Analysis of the composés nouveaux en 13 C ‐ RMN. I. (±) Alkyl ‐ 1 ‐ déséthyl ‐ 3 ‐ émétine à jonction B / C cis ou trans. In: Helvetica Chimica Acta . Volume 60, number 7, November 1977, pp. 2122-2134, doi: 10.1002 / hlca.19770600703 , PMID 200588 .
- E. Melchior: Therapeutic use of emetine. In: Munich medical weekly. Volume 113, Number 28, September 1971, pp. 1235-1236, PMID 5110069 .
Individual evidence
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ F. Magendie, PJ Pelletier: Recherches chimiques et physiologiques sur l'ipécacuanha. In: Annales de chimie et de physique . 4 (1817), pp. 172-185. (Digitized version)
- ^ François Magendie . Formulaire pour la preparation et l'emploi de plusieurs nouveaux médicamens. Tels que la noix vomique, la morphine, l'acide prussique, la strychnine, la vératine, les alcalis des quinquinas, l'iode, etc., etc., etc. by F. Magendie, Membre de l'Académie royale de médecine, Médecin du Bureau central d'administration aux hôpitaux et hospices de Paris, etc., etc. Paris: Méquignon-Marvis 1821, pp. 24–33: Emétine. Emétine pure. (Digitized version)
- ↑ Regulations for the preparation and use of some new medicines such as the crow's eyes, morphine, prussic acid, strychnine, veratrine, china alkaline, iodine uma, iodine uma by F. Magendie. From the French. Leop. Voss, Leipzig 1822, pp. 28–37: Emetin. Pure emetine. (Digitized version)
- ^ ED Vedder: An experimental study of the action of ipecacuanha on amoebae. In: Transactions of the 2nd Biennal Congress, Far-Eastern Association of Tropical Medicine , p 87, 1912 (abstract in: J Trop Med Hyg 15: 313-314).
- ^ HH Anderson: Emetine in Amebiasis. In: California and Western Medicine . Volume 40, Number 3, March 1934, pp. 198-199, PMID 18742814 , PMC 1658975 (free full text).
- ↑ a b R. Rohkamm, G. Reimann, K. Ricker: Toxische Myopathie durch Emetin. In: H. Gänshirt, P. Berlit, G. Haack (editor): Cardiovascular diseases and nervous system neurotoxicology problems of brain death. Negotiations of the German Society for Neurology (58th annual conference from September 19-22, 1984 in Heidelberg), Vol 3. Springer, Berlin, Heidelberg, doi: 10.1007 / 978-3-642-46521-5_112 .
- ↑ E. Teuscher: Biogenic Medicines. 5th edition. Wissenschaftliche Verlagsgesellschaft, 1997. ISBN 3-8047-1482-X . P. 329.
- ↑ Shu Yang, Miao Xu et al .: Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. In: Cell Discovery , Vol 4, Article number: 31 (2018), Nature
- ^ PF Uzor: Recent developments on potential new applications of emetine as anti-cancer agent. In: EXCLI Journal . Volume 15, 2016, pp. 323–328, doi: 10.17179 / excli2016-280 , PMID 27366142 , PMC 4928012 (free full text).
- ↑ T. Dingermann, Karl Hiller, G. Schneider, I. Zündorf: Schneider drug drugs. 5th edition. Elsevier, 2004. ISBN 3-8274-1481-4 . P. 473 f.
- ^ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, T. Groemer; G. Spitzer, K. Liedl, E. Gulbins, P. Tripal: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . tape 6 , no. 8 , 2011, p. e23852 , doi : 10.1371 / journal.pone.0023852 .
- ↑ External identifiers or database links for emetine dihydrochloride : CAS number: 316-42-7, EC number: 206-259-8, ECHA InfoCard: 100.005.692 , GESTIS substance database : 510718 , PubChem : 3068143 , ChemSpider : 2328125 , Wikidata : Q27290698 .
- ↑ External identifiers or database links for emetine dihydrochloride-5-water : CAS number: 79300-07-5, PubChem : 71587289 , ChemSpider : 32697461 , Wikidata : Q27236300 .
- ↑ External identifiers or database links for emetine dihydrochloride-7-water : CAS number: 79300-08-6, PubChem : 71587290 , ChemSpider : 32699961 , Wikidata : Q27281245 .
- ↑ External identifiers of or database links to (+) - emetine dihydrochloride-x-water : CAS number: 313222-95-6, EC number: 621-896-5, ECHA InfoCard: 100.150.707 , PubChem : 71311519 , ChemSpider : 49072958 , Wikidata : Q82695180 .
- ↑ External identifiers or database links for emetine dihydrobromide-4-water : CAS number: 13903-43-0, PubChem : 197921 , ChemSpider : 171305 , Wikidata : Q82905560 .
- ↑ European Pharmacopoeia 9.0 (2017) p. 2349: "Emetine hydrochloride pentaydrate ( Emetini hydrochloridum pentahydricum )".
- ↑ European Pharmacopoea 7.0 (2011) p. 1914: "Emetine hydrochloride heptahydrate ( Emetini hydrochloridum heptahydricum )".