Enterobactin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
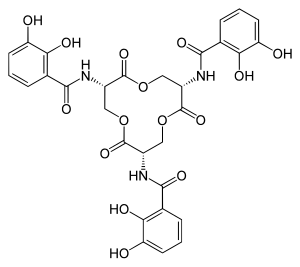
|
|||||||||||||
| General | |||||||||||||
| Surname | Enterobactin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 30 H 27 N 3 O 15 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 669.55 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
202-203 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Enterobactin is a chemical compound . As a natural substance, it belongs to the siderophores (iron carriers). It is mainly found in gram-negative bacteria such as Salmonella typhimurium , Escherichia coli and Klebsiella pneumoniae .
Biological importance
Enterobactin, which is a cyclic trimer of N - (2,3-dihydroxybenzoyl) - L -serine, is able to coordinate iron . It forms extremely stable chelate complexes with iron ions (Fe 3+ ) . In the complex, the iron is bound octahedrally by the six oxygen atoms of the three catechol residues. This leads to a very high complex formation constant of K = 10 52 (for comparison: the constant of the complexation of Fe (III) with EDTA is 10 25 ). The affinity for Fe 2+ is significantly lower, a reduction in the central atom therefore causes the iron to be dissolved out of the complex. This property is used to transport iron within the bacteria. Enterobactin is also used to fix vanadium .
In Salmonella, enterobactin competes with the iron transporters of the host organism .
biosynthesis
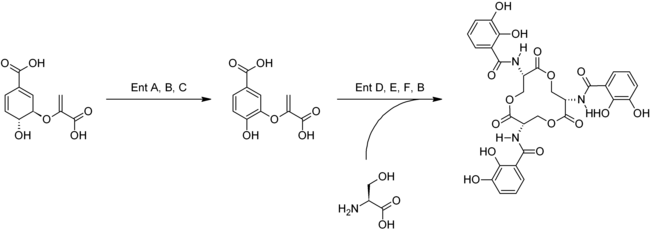
The multi-stage, enzymatic biosynthesis takes place via the shikimic acid route and starts from chorismic acid . This is converted into isochorismate as a chorismate by isochorismate synthase (a mutase ) . This is converted to 2,3-dihydroxybenzoate by a number of other enzymes . This intermediate is reacted with the proteinogenic amino acid L serine for N - - (2,3-dihydroxybenzoyl) L reacted -serine. This is dimerized and forms enterobactin.
Individual evidence
- ↑ a b c Entry on Enterobactin. In: Römpp Online . Georg Thieme Verlag, accessed on June 25, 2014.
- ↑ Enterobactin data sheet from Sigma-Aldrich , accessed on June 25, 2014 ( PDF ).
- ^ W. Ternes: Biochemistry of the elements: Inorganic chemistry of biological processes , Springer-Verlag, 2013, ISBN 978-3827430199 , p. 75.
- ^ P. Berg, M. Singer: Dealing with Genes: The Language of Heredity , University Science Books, 1st edition, 1992, ISBN 978-0935702699 , pp. 108ff.
- ↑ H. Sinell: Introduction to Food Hygiene , 4th, revised edition, Enke Verlag, 2003, ISBN 978-3830440956 , p. 22.
- ↑ EC 5.4.4.2 .
- ↑ Daniel L. Purich, R. Donald Allison: The Enzyme Reference , Academic Press, 2003, ISBN 978-0125680417 , page 265 486f.