Fluorenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fluorenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 8 O | |||||||||||||||
| Brief description |
yellow scales |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 180.21 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.9 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
84 ° C |
|||||||||||||||
| boiling point |
342 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.6309 (99 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
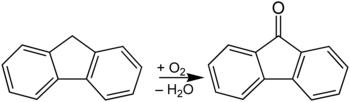
Fluorenone is a derivative of fluorene (a polycyclic aromatic hydrocarbon ) and belongs to the group of aromatics and ketones . The difference to fluorene is the keto group on the five-membered ring (in the 9-position).
history
Derivatives of fluorenone were the first drugs worldwide with a proven effectiveness against viruses in rodents ( Tiloron , since around 1970). However, clinical use has been discontinued due to a lack of effectiveness in humans.
Extraction and presentation
Fluorenone is produced by the oxidation of fluorene in a basic medium:
Furthermore, it is produced (like fluorene) in low concentrations when petrol and diesel are burned.
properties
The pure substance is a flammable, yellow, crystalline solid substance. Fluorenone has a striking, intense color in solution, which explains its use in chromatography . The darker (yellow) color compared to fluorene results from the expansion of the aromatic system to the oxygen atom (through conjugated double bonds ).
use
Fluorenone is used as a dye in column chromatography. Some derivatives were used as anti-viral drugs ( antivirals ) before . Research is currently underway to develop new chemotherapeutic and virostatic agents made from fluorenone (analogous to tiloron ). Appropriate substituents on the molecule should maximize effectiveness and minimize side effects. In addition, the growth regulator chlorflurenol is synthesized from fluorenone.
safety instructions
In contrast to fluorene, fluorenone is not acutely toxic. It's a strong mitogen . Therefore suspected carcinogenic properties cannot be proven. The fluorene derivative 4-acetylaminofluorene (AAF), on the other hand, has a carcinogenic effect on animals and humans.
Web links
- Oxidation of fluorene (PDF file; 15 kB, Dutch)
- 9-fluorenone-carboxamides (PDF file; 296 kB)
- Stavros Kromidas: Two tests to characterize RP phases. In: Laborpraxis Online . May 3, 2006, accessed July 15, 2017 .
Individual evidence
- ↑ a b c d e f Entry on Fluoren-9-one in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b Entry on Fluoren-9-one. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-258.
- ↑ HE Kaufman, YM Centifanto, ED Ellison, DC Brown: Tilorone hydrochloride: human toxicity and interferon stimulation. In: Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. Volume 137, Number 1, May 1971, pp. 357-360, doi : 10.3181 / 00379727-137-35576 , PMID 5581674 .
- ↑ S. Ekins, MA Lingerfelt, JE Como, AN Freiberg, JC Mirsalis, K. O'Loughlin, A. Harutyunyan, C. McFarlane, CE Green, PB Madrid: Efficacy of Tilorone Dihydrochloride against Ebola virus infection. In: Antimicrobial agents and chemotherapy. Volume 62, number 2, 02 2018, p., Doi : 10.1128 / AAC.01711-17 , PMID 29133569 , PMC 5786809 (free full text).
- ↑ T. Cavlar, T. Deimling, A. Ablasser, KP Hopfner, V. Hornung: Species-specific detection of the antiviral small-molecule compound CMA by STING. In: The EMBO Journal . Volume 32, number 10, May 2013, pp. 1440–1450, doi : 10.1038 / emboj.2013.86 , PMID 23604073 , PMC 3655471 (free full text).
- ↑ Sascha Nikolov: Early effects of genotoxic and non-genotoxic carcinogens (fluorene) on the cell division of various non-target tissues in young male rats . Jena 2002, DNB 966335503 , urn : nbn: de: gbv: 27-dbt-000806-2 (dissertation, University of Jena).
- ↑ Udo Fuchs: Influence of various cell toxins, mitogens and heavy metals on the cytochrome P450-dependent monooxygenase function in liver cell transplant-containing spleens compared to the liver in rats . Jena 2005, DNB 975818406 , urn : nbn: de: gbv: 27-dbt-004156-2 (dissertation, University of Jena).