Flutianil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
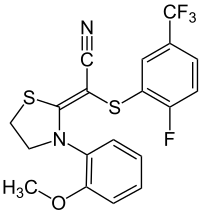
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Flutianil | ||||||||||||||||||
| other names |
(2 Z ) -2 - [(2-Fluoro-5-trifluoromethyl) phenyl] thio-2- [3- (2-methoxyphenyl) -2-thiazolidinylidene] acetonitrile |
||||||||||||||||||
| Molecular formula | C 19 H 14 F 4 N 2 OS 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 426.45 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
178-179 ° C |
||||||||||||||||||
| boiling point |
245–255 ° C (decomposition) |
||||||||||||||||||
| Vapor pressure |
1.530 10 −7 Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Flutianil is a chemical compound from the group of thiazolidines .
Extraction and presentation
Flutianil can be obtained by a multi-stage reaction starting from a suitable substituted aniline . Diazotization and conversion of the diazonium salt into a dithiocarbonate leads to an intermediate product, which is deprotected and cyanomethylated to a nitrile . Reaction with 2-methoxyphenyl isothiocyanate leads to a further intermediate product that is ultimately cyclized with 2-dibromoethane .
properties
Flutianil is a white solid that is practically insoluble in water.
use
Flutianil is used as a fungicide and was launched by Otsuka in July 2008.
safety instructions
Before the harmonized classification, flutianil was discussed with regard to a classification with suspected carcinogenic effects and reproductive toxicity (H351 and H361).
Individual evidence
- ↑ a b c d e f g h i j k l m n o EFSA : Conclusion on the peer review of the pesticide risk assessment of the active substance flutianil . In: EFSA Journal . tape 12 , no. 8 , August 2014, p. 3805 , doi : 10.2903 / j.efsa.2014.3805 .
- ↑ a b Entry on Flutianil at Toronto Research Chemicals , accessed on January 6, 2020 ( PDF ).
- ↑ entry on flutianil (ISO); (2Z) - {[2-fluoro-5- (trifluoromethyl) phenyl] thio} [3- (2-methoxyphenyl) -1,3-thiazolidin-2-ylidene] acetonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on November 5, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds: Herbicides . John Wiley & Sons, 2012, ISBN 978-3-527-32965-6 , pp. 896 ( limited preview in Google Book Search).
