Photoinitiator
Photoinitiators are chemical compounds that break down in a photolysis reaction after the absorption of ( UV ) light and thus form reactive species that can start (initiate) a reaction (usually a polymerization ). The reactive species are radicals or cations .
Photoinitiators are usually classified according to the type of reactive species (cationic, radical), sometimes also according to their molar mass (low molecular weight or polymer).
use
Photoinitiators are components of radiation-curing lacquer and resin formulations that can be cured in a fraction of a second by exposure to UV light. They are used, for example, in diazotype , stereolithography and multi-jet modeling . The largest market segments for coatings are furniture and parquet lacquering as well as printing inks. Photoinitiators can also be used in LCD production.
A radiation-curing system (e.g. a lacquer) can be polymerized either with the aid of an initiator or directly by electron beams . UV radiation, which would be strong enough to initiate the radical polymerization of the monomers (or oligomers) directly, has only a small penetration depth and can therefore not be used. Instead, radicals from photoinitiators are generated with the help of longer-wave UV radiation (254 to 400 nm), which has a greater penetration depth.
When choosing the photoinitiator, the absorption spectrum of the initiator must be compared with the emission spectrum of the light source.
Classification according to reactive center
Free radical photoinitiators
Free radical chain reaction photoinitiators are divided into two types. Type 1 photoinitiators generate radicals directly in a photofragmentation, mostly through alpha cleavage . The radical formed then triggers the chain polymerization . Type 2 photoinitiators, on the other hand, abstract a hydrogen atom from a neighboring molecule. This then triggers the chain polymerization. Usually tertiary amines are added, as these form particularly effective starter radicals and thus increase reactivity.
Photoinitiators that are frequently used in industry are derived from α-hydroxy, α-alkoxy or α-amino aryl ketones or from acylphosphine oxides. There are also isolated applications for photolabile aliphatic azo compounds, which can decompose both thermally and photochemically.
Cationic photoinitiators
Cationic photoinitiators produce a Brönsted or Lewis acid when they decompose. They can be classified according to thermal stability ( thermolatent or thermally unstable).
Thermal latent photoinitiators
Radicals that initiate polymerization can be generated by simple compounds such as peroxides or disulfides by irradiation with homolytic bond cleavage. For industrial applications, however, the photoinitiator used must be dissolved in monomers for a long time (months to years) before use , without polymerization (gelation) occurring. When irradiated, it has to start the polymerization with a high quantum yield and free of side reactions. For this reason, AIBN, peroxides or disulfides are hardly suitable for industrial applications. For this reason, thermolatent photoinitiators have been developed which are stable to heat ( thermal latency ) and can therefore be stored dissolved in monomers for a long time in the absence of light . They also have optimized absorption spectra so that they can be used under various application conditions.
Thermolatent photoinitiators are used in particular for the cationic polymerization of epoxy resins , with cycloaliphatic epoxy resins being more reactive than glicidyl epoxy resins. Most of such thermolatent photoinitiators are onium compounds .
history
In the 1970s, the aim of industrial paint research was to establish radiation curing as an economical alternative as an alternative to energy-intensive thermal curing (which requires the operation of large ovens ) . The first important discovery in this direction was made by employees of the American Can Company . They found that the photosensitivity of aryldiazonium salts can be used for the cationic polymerization of epoxides:
The aryldiazonium salt decomposes with formation of the Lewis acid boron trifluoride , which initiates the cationic polymerization .
However, since aryldiazonium salts are thermally unstable, adding initiator to monomer (a formulation ) often leads to gelling after a few hours, even in the absence of light . Another disadvantage of the aryldiazonium salts is the formation of (gaseous) nitrogen , which can lead to defects in the cured paint. Therefore, although the aryldiazonium salts are not of commercial importance, the discovery led to the realization that photosensitive, organic compounds are able to initiate cationic polymerization and thus to intensive research in this direction. The onium compounds were soon discovered as efficient photoinitiators, and these are still in use today. Onium compounds no longer have the disadvantages of the aryldiazonium salts, but have good thermal stability (i.e. thermal latency) and do not release any gaseous products when they are split. They also have intense absorption in the short-wave UV range and a good quantum yield . First the diaryliodonium salts were discovered, followed later by the thermally particularly stable triarylsulfonium salts . These also had the advantage that they were already being produced as corrosion inhibitors at that time and were therefore available at low cost.
Traditional cationic initiators ( Lewis or Brönsted acids ) lead to instant polymerization. Industrially, however, a storable formulation is desired, the polymerization of which can be triggered by an external stimulus (UV radiation). The commercial use of cationic crosslinking was therefore only possible with the development of onium photoinitiators.
Industrial use
In principle, almost any cationically polymerizable polymer can be produced with onium compounds as an initiator, but mostly the onium compounds are used to produce crosslinked polymers, mostly epoxides. By means of photocationic epoxy polymerisation, both high polymerisation rates can be achieved even at low temperatures, as can precise crosslinking through selective irradiation. The main areas of application are therefore photo-curing coatings, coatings in microelectronics and stereolithography, printing inks and adhesives.
There are numerous classes of thermolatent photoinitiators, some typical representatives are listed in the following table. As can be seen, most of them belong to the class of onium compounds.
| Class name | Structural formula |
|---|---|
| Aryldiazonium |

|
| Diaryliodonium |

|
| Triarylsulfonium |
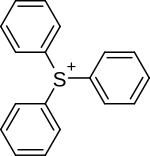
|
| Ferrocenium |

|
| Phenacyl triphenylphosphonium |

|
| N -alkoxypyridinium |

|
| Pyrylium |
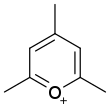
|
| Thiapyrilium |

|
Division according to molar mass
While photoinitiators are usually low molecular weight , there are also (cationic) photoinitiators that are bound to a polymer. One advantage is that such a (high molecular weight) photoinitiator does not migrate into its environment and is therefore non-toxic. Another advantage is that it starts a polymerization reaction from the polymer already present and thus leads to a higher degree of crosslinking.
Health hazards
In 2009 several authorities for food safety in dry foods z. B. Grain products discovered photoinitiators. Due to the very small size of the photoinitiator molecules, they can migrate from the printed side of the food packaging through several layers of packaging (e.g. paper, cardboard or plastic) into the food. The limit values were mostly undercut during control examinations, but since there is a separate limit value for each type of photoinitiator and no addition takes place, a considerable amount of substances can be present in the food without any complaints. The health effects are mostly viewed as critical. Two photoinitiators, benzophenone and 4-methylbenzophenone, have been classified as harmful in animal experiments, others have not yet been tested. These two substances can cause liver and kidney ulcers. The permissible limit is 0.6 micrograms per kilogram of food. The limit value was slightly exceeded for some tested foods.
literature
- Entry on photoinitiators. In: Römpp Online . Georg Thieme Verlag, accessed on December 19, 2014.
- R. Schwalm: UV Coatings - Basics, Recent Developments and New Applications. Elsevier 2007, ISBN 978-0-444-52979-4 .
- Dieter Stoye, Günter Beuschel: Coating resins: chemistry and properties and applications . Hanser Verlag, 1996, ISBN 3-446-17475-3 , p. 93 ( limited preview in Google Book search).
Web links
- Lower Saxony State Office for Consumer Protection and Food Safety examines packaging
- Food: Poisons in focus
Individual evidence
- ^ Research award 2005 from Ciba Specialty Chemicals for new photoinitiator technology for LCDs
- ↑ a b (2) Pieke, S. Experimental studies on the efficient crosslinking of surface coatings with UV radiation; KIT Scientific Publishing, 2010; pp. 10-22. (3) Allen, NS Journal of Photochemistry and Photobiology A: Chemistry 1996, 100, 101-107.
- ↑ Yusuf Yağcı, Ivo Reetz: Externally stimulated initiator system for cationic polymerization . In: Progress in Polymer Science . 23, No. 8, December 1998, pp. 1485-1538. doi : 10.1016 / S0079-6700 (98) 00010-0 .
- ↑ a b c d e f Verena Görtz: Benzothiazolium salts as photoinitiators for cationic polymerisation 2005. online ( Memento of the original from December 19, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ W. Arthur Green: Industrial Photoinitiators: A Technical Guide ( limited preview in Google Book Search).
- ↑ SR-online: Printing chemistry in food ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ Extent of migration of printing ink components from packaging materials into food (PDF; 859 kB), May 29, 2011.

