Chain polymerization
Chain polymerization (in German after IUPAC chain polymerization , often referred to as polymerization in German-speaking countries and not in accordance with IUPAC ) is a collective term for chain reactions in which the same or different monomers are continuously and exclusively linked to a growing polymer chain . The growing chain has at least one active site , where the addition takes place of the monomers. Depending on the structure of the active centers, the polymerizations can be divided into radical, cationic, anionic and coordinative chain polymerizations. In chain polymerizations, no by-products are split off during the addition.
No chain polymerizations are polyadditions and polycondensations , in which the polymerization takes place in step growth and thus reactions between molecules of all degrees of polymerization take place.
Mechanisms
Free radical chain polymerization
Free-radical polymerization essentially comprises four sub-steps:
- Disintegration of an initiator
- Initial reaction in which the active center is formed.
- Growth reaction in which the macromolecular chain grows in a chain reaction (repeated addition of monomers ), and
- Termination reaction in which the growth of the chain is irreversibly terminated by disproportionation reactions or combinations.
mechanism
An added initiator breaks down with the formation of two radicals R • . The disintegration is usually promoted by increased temperature (40–90 ° C). With suitable initiators, the decomposition can also be initiated photochemically , for example .
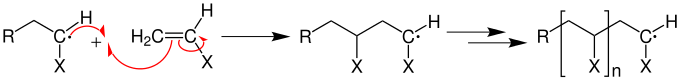
At the start of the chain , the radical breaks the multiple bond (for example a C = C double bond of a vinyl group ) and generates a primary radical that is capable of growth:
Monomers are now constantly attached to the primary radical in a growth reaction with a low activation energy .
The meeting of two radicals, i.e. a combination, causes a chain break.
Furthermore, chain termination can be brought about by disproportionation .
In addition, some control over the molar mass can be achieved by adding chain transfer agents to the polymerization .
Kinetics of radical chain polymerization
Equations for the reaction rates can be formulated for each of the partial reactions of radical polymerization . Knowing these relationships makes it possible to control the average degree of polymerization of a polymer formed and to interpret some of the effects occurring during the polymerization.
initiation
The speed of initiation depends on the concentration of the initiator and the corresponding speed coefficient :
The initiator disintegration does not necessarily provide completely effective radicals R *, since some of them do not cause a chain start, but react or recombine in some other way. Accordingly, a factor is introduced that reflects the effectiveness of initiator disintegration for chain start:
Chain growth
The rate of chain growth, i.e. monomer consumption, depends on the monomer concentration , the concentration of the polymer radicals and the corresponding rate coefficient :
Recombination
The speed of recombination, i.e. the consumption of polymer radicals P *, depends on the square of their concentration and of course on the coefficient :
transmission
The speed of the transfer depends on the concentration of the carrier , the concentration of polymer radicals and the coefficient :
Root I law
From the given rate laws one can derive a formula for the growth rate. This only applies to medium sales, but then basically indicates the gross reaction speed:
Mayo equation
The number average of the degree of polymerization can also be determined. It results from the ratio of the growth rate to the rates of all reactions at which the growth breaks off:
The American chemist Frank R. Mayo derived a formula from this with which one can either calculate the transfer constant for a mixture of monomer and carrier, or the average degree of polymerisation of the resulting polymer if one mixes specific amounts of monomer and carrier:
Therein is the number average of the degree of polymerization in the absence of a carrier.
Synthesis course
In the simplest case, the course of a synthesis via radical polymerization is only observed via the consumption of the monomers (as the gross reaction rate) and is determined by several factors:
- The addition of monomers to an active chain takes place very quickly and is mainly determined by the diffusion of the monomers to the active center of the growing chain.
- The lifetime of a growing chain until the termination reaction is short compared to the period of the entire synthesis.
- Over the entire period of the synthesis, new start reactions take place continuously due to the ongoing decomposition of the initiator. The decomposition reaction has a very high activation energy and is therefore temperature-dependent.
- Free-radical polymerization is exothermic , since the weaker π-bonds of the double bond of the monomer are converted into stronger σ-bonds of the macromolecule.
The implementation of a mixture of monomer and initiator ( bulk polymerization , also called mass process) in a closed reactor ( batch process ) seems to be the simplest case:
With a very low conversion (<0.01%), initiator radicals and oligomeric radicals are increasingly formed. The gross reaction speed increases rapidly and changes into a continuous reaction process: The gross reaction speed decreases continuously because the concentration of the monomers falls due to the increasing concentration of the macromolecules. The concentration of the growing chains (chain radicals) is, however, almost constant (quasi-stationary state according to Bodenstein's principle of quasi-stationaryity ).
From the range of 20 to 40% conversion of the monomers, the properties of the reaction mixture change significantly, since the monomers also act as solvents for the macromolecules. The viscosity of the reaction mixture increases and the polymer radicals are prevented from diffusion because of their size. As a result, fewer termination reactions occur due to the combination of two growing chains. The initiator continues to break down and form new growing chains that react with the still mobile monomers. The polymerization is subject to a self -acceleration , which is also known as the gel or Trommsdorff-Norrish effect . Since the polymerization is exothermic and a higher temperature promotes initiation, there is a risk of explosion, especially if the heat cannot be removed from the reactor. The glass effect occurs from around 80% conversion: the polymers and chain radicals are now immobile, so that the reaction mixture slowly solidifies ( glass state ). The remaining monomers are increasingly immobile in the reaction mixture, which is why the overall reaction rate progresses more and more slowly. Complete conversion of the monomers is i. d. Usually not reached. Because of the gel effect, substance polymerizations on an industrial scale are only carried out to a limited extent and broken off, since only insufficient heat can be dissipated. Other technical processes offer alternatives , in the simplest case the use of solvents in order to reduce the gel effect and avoid the glassy state.
A special case of radical polymerization is popcorn polymerization .
Controlled radical polymerization
By adding 'control reagents' it is possible to suppress the termination reactions of the radical polymerization by bringing the growing chains into a dynamic equilibrium with a “sleeping” state of the active centers.
While the molecular weight and polydispersity can hardly be controlled in conventional free-radical polymerizations, controlled free-radical polymerization (CRP) allows the production of polymers with small polydispersity (narrow molar mass distribution) and, for example, low molecular weight.
In the case of controlled radical polymerization, all initiations take place at the beginning of the synthesis, since the growth reactions take place slowly on average. At any point in the synthesis there is only a very low concentration of particles with reactive chain ends, since most of the initiated chains are in the “sleeping” state. Most of the initiated particles go through the entire synthesis process with temporary “growing” and temporary “sleeping”, ie until the monomers are used up. This allows, for example, a subsequent addition of a comonomer B, which leads to the formation of block copolymers (AAAABBB).
Some control reagents Inifer reagents (ger .: ini ator trans fer agent) called and disintegrate in an active radical (the initiator of polymerization) and a fairly stable, persistent radical which can not initiate, but by addition to an active site, the The chain is reversibly transferred to a "sleeping" state. Examples of such a process are atom transfer radical polymerization (ATRP) and nitroxide-mediated radical polymerization (NMRP). Another method is RAFT polymerization (reversible addition-fragmentation chain transfer), in which a conventional initiator is used and an adduct radical is formed as a “dormant” state.
Cationic chain polymerization
An acid such as fluoroboric acid , for example, acts as a starter , which protonates a monomer unit on the double bond in the start reaction .
In the growth reaction, the cation resulting from the start reaction is added to a further monomer, which in turn creates a cation.
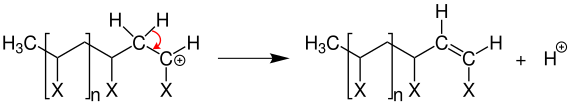
A termination reaction , as in free radical polymerization , does not occur. However, a proton can be transferred to a new monomer unit through an elimination reaction .
This stops the chain from growing and a proton is available for another start reaction.
Anionic chain polymerization
Here the growing chain represents an anion , such a reaction is started by lithium organyls or Grignard compounds . Often no transfer or termination reaction can be formulated, one then speaks of a living polymerization . As a result, polymers with a very uniform chain length ( polydispersity close to one) can often be obtained. Anionic polymerization reactions are generally sensitive to the slightest traces of water and therefore require very carefully absolute starting materials.
The preparation of polyoxymethylene (POM) may be of a anionic chain polymerization of formaldehyde carried out:
The polymerization is initiated by a base such as sodium methoxide .
The growth takes place as a chain polymerization:
Termination occurs due to traces of water or methanol:
Coordinative chain polymerization
The catalysts are transition metal compounds whose structure has to be characterized in such a way that a central atom (the metal ion) is surrounded by ligands in such a way that a monomer and the polymer chain can attach (coordinate) to it. The principle is based on an activation of the monomers due to the interaction of the monomer with the metal. The double bond in the monomer is thereby weakened and the addition of a second monomer is initiated. To stabilize the complex compound formed in this way, the monomer is inserted into the existing polymer chain and a further monomer is added, etc. The polymerization reaction is thus initiated. The process is also called insertion polymerization .
The advantage of coordination polymerization is that, depending on the choice of catalyst and monomers, the tacticity of the resulting polymer can be controlled, which can have a significant influence on the polymer properties.
There are different types of coordination polymerizations with different mechanisms. The most important one is the Ziegler-Natta polymerisation , named after its discoverer , which allows, for example, ethene to be converted into linear high-density polyethylene (PE-HD) at low temperatures and low pressures . Of great importance also is the polymerization with metallocene - catalysts .
More specific types of coordination polymerization such as ring-opening metathesis polymerization (ROMP) are used in the production of special polymers that are produced by ring opening and linking of cyclic monomers by transition metal catalysts .
Technical procedures
- Bulk polymerization (also called mass polymerization or bulk polymerization): monomer reacts with the initiator in pure form without solvent
- Solution polymerization monomer and polymer in: solvent dissolved
- Precipitation polymerization: monomer dissolved in solvent, polymer precipitates
- Emulsion polymerization : Monomer dissolved in water by emulsifier , polymer precipitates
- Suspension polymerization : Monomer suspended in water (small drops) by stirring and stabilizers, polymer precipitates
- Gas phase polymerization: gaseous monomer keeps polymer granules in fluidized bed
- Polymerization of monomolecular layers according to Langmuir-Blodgett
literature
- JMG Cowie: Chemistry and Physics of Synthetic Polymers. , Vieweg, 2 ed., 1991.
- K. Matyjaszewski , TP Davis: Handbook of Radical Polymerization. , Wiley, 2002.
- B. Tieke: Makromolekulare Chemie , Wiley-VCH, 2002.
- HG Elias: Makromoleküle , Wiley-VCH, 2002.
Web links
- Polymerization process in Chemgapedia
- 2-dimensional animation of a radical chain polymerization using polyvinyl chloride (PVC)
Individual evidence
- ↑ Entry on chain polymerization . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00958 Version: 2.3.2.
- ↑ Entry on polymerization. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Bernd Tieke, Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, pp. 60ff.
- ↑ a b c d e Lechner, Manfred D .; Gehrke, Klaus; Nordmeier, Eckhard: Macromolecular chemistry: a textbook for chemists, physicists, materials scientists and process engineers . 4th edition. Birkhäuser, Basel; Boston, Mass .; Berlin 2010, ISBN 978-3-7643-8890-4 , pp. 160–170 ( limited preview in Google Book Search).
- ^ Karlheinz Biederbick: Kunststoffe , 4th edition, Vogel-Verlag, Würzburg, 1977, p. 60, ISBN 3-8023-0010-6 .

![{\ displaystyle \ mathrm {I \ \ {\ xrightarrow [{}] {\ Delta}} \ 2 \ R ^ {\ bullet}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/71e2f46169865998fc9bf5456ea321ccc3c666cd)




![[I]](https://wikimedia.org/api/rest_v1/media/math/render/svg/5962300a54e8ce8b5761dac9a5fbbca450c2ce0f)

![v _ {{{\ text {ini}}}} = - {\ frac {{\ mathrm {d}} [I]} {{\ mathrm {d}} t}} = k _ {{{\ text {ini} }}} \ cdot [I]](https://wikimedia.org/api/rest_v1/media/math/render/svg/fc9a6708c33e270cdbba72a7c7580d20c4dfdff8)

![{\ displaystyle {\ frac {\ mathrm {d} [R *]} {\ mathrm {d} t}} = 2f \ cdot k _ {\ text {ini}} \ cdot [I]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/71fdbf4a7c8eb8882a8e0488c7d13d8ad57d0a86)

![[M]](https://wikimedia.org/api/rest_v1/media/math/render/svg/e5ca74e595b2281c0aef1897ecafa282d1f182e2)
![[P *]](https://wikimedia.org/api/rest_v1/media/math/render/svg/ed5bec53a19741331e458e7bc22d309d6de94ad4)

![{\ displaystyle v _ {\ text {w}} = - {\ frac {\ mathrm {d} [M]} {\ mathrm {d} t}} = k _ {\ text {w}} \ cdot [M] \ cdot [P *]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/314c9d1c09d1e025c0144ac3e137016fd7ab0589)


![{\ displaystyle v _ {\ text {stop}} = - {\ frac {\ mathrm {d} [P *]} {\ mathrm {d} t}} = k _ {\ text {stop}} \ cdot [P * ] ^ {2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/15f9b672f511b4e70a62ac1420ffc9a3eed0b7dd)

![[Trans]](https://wikimedia.org/api/rest_v1/media/math/render/svg/f1bd4cc71351b5de3f2fea5356fd453b3bd92692)

![{\ displaystyle v _ {\ text {trans}} = k _ {\ text {trans}} \ cdot [P *] \ cdot [Trans]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/68bc53326acd969c2e811c9d1f8400e25de2cb34)
![{\ displaystyle v _ {\ text {w}} = k _ {\ text {w}} \ cdot {\ sqrt {2f \ cdot {\ frac {k _ {\ text {ini}}} {k _ {\ text {stop} }}}}} \ cdot [M] \ cdot {\ sqrt {[I]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6382f540522f010efa8a9393263e17bdce5e5fad)



![{\ displaystyle {\ frac {1} {{\ overline {X}} _ {n}}} = {\ frac {1} {{\ overline {X}} _ {n, 0}}} + {\ frac {k _ {\ text {trans}}} {k _ {\ text {w}}}} \ cdot {\ frac {[Trans]} {[M]}} = {\ frac {1} {{\ overline {X }} _ {n, 0}}} + C \ cdot {\ frac {[Trans]} {[M]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c9ae4a9781b49ca3520e74761ef4037fa1785458)





![{\ displaystyle \ mathrm {{\ xrightarrow [{}] {+ H_ {2} C {=} O}} \ {\ xrightarrow [{}] {+ H_ {2} C {=} O}} \ {\ xrightarrow [{}] {+ H_ {2} C {=} O}} \ H_ {3} C {-} O ({-} CH_ {2} {-} O) _ {\ mathit {n}} { -} CH_ {2} {-} O ^ {-} \ Na ^ {+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/68d08f7a2472ca43d15a1a4e9e7a425e7674d4b3)
