Double bond
A double bond is a form of covalent bond that involves four bonding electrons . The two atoms are thus connected to one another via two bonding electron pairs . In structural formulas, double bonds are represented as two parallel lines (=) between the connected atoms.
Many atoms can form double bonds with one another. The most common functional groups or substance classes with double bonds are the alkenes (C = C), carbonyls (C = O), sulfoxides (S = O), imines (C = N) and the azo group (N = N).
Double bonds are stronger and shorter than single bonds. The bond order is two. Double bonds are electron rich, which makes them reactive.
history
The representation of the double bond by two double lines was introduced by Joseph Loschmidt . Around 1865 August Wilhelm von Hofmann introduced the endings -an, -en, -in to denote saturated and unsaturated alkanes.
Detailed description

The bonding relationships of a molecule of the 2nd period with a double bond can be described with sp 2 - hybrid orbitals . The neighboring atoms of such an atom with sp 2 hybridization are in one plane (the molecule is planar). The angles between the bonded atoms are mostly close to 120 ° (see VSEPR model), the distance between the bonding atoms is around 1.34 Å. The remaining electron is in an orbital with pure p-character perpendicular to the molecular plane. Each of the two atoms that are connected by the double bond each have such a singly occupied p orbital . The combination of these two creates a bonding π orbital and an antibonding π * orbital.
This type of double bond is not limited to the 2nd period, but different bonding patterns are found in double bonds between elements of the higher periods. The reasons for this are, on the one hand, generally lower homonuclear binding energies for larger atoms and, on the other hand, a lower hybridization tendency of the s and p valence orbitals. In certain cases, the coincidence of these phenomena means that the energy that is required to bring the molecular fragments on which the double bond system is based into a bondable state can no longer be applied by the subsequently released binding energy. In such cases, either these fragments (i.e., carbene-homologous or analogous molecules) are obtained as isolable compounds or the dimerization occurs via a double Lewis acid-base reaction and results in two donor-acceptor bonds (a double dative bond). Such a double bond, which is observed, for example, in the case of the higher carbon homologues, can be recognized from a characteristic structure (trans-bent bending of the substituents) and the low energy required to separate the bond. The dissociation energy can even be significantly smaller than for a normal covalent single bond. Typical examples of such double bond systems are distannenes (R 2 Sn = SnR 2 ) and Diplumbene (R 2 Pb = PbR 2 ).
The energy gap between the bonding π and antibonding π * orbital is usually smaller than the energy gap between a corresponding σ and an antibonding σ * orbital. In the case of conjugated double bonds , the excitation energy drops so far that visible light can be sufficient to lift an electron into an empty orbital. The greater the number of conjugated double bonds, the less energy (longer-wave light) is required (see also: Particles in the box ). In the case of carotene with eleven conjugated double bonds, blue light is absorbed and the molecule appears orange (the complementary color orange arises from the subtraction of the blue component from the radiated white light).
Bond lengths and bond angles
The bond lengths of double bonds between two atoms in non-conjugated systems are shorter than those of the corresponding single bonds between the same atoms.
Bond lengths and bond angles of selected double bonds 
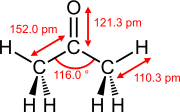

Ethene (ethylene) acetone Dimethyl sulfoxide
nomenclature
The rotation around a double bond is not easily possible. If both binding partners have two different substituents , a distinction is made between cis and trans isomers with regard to their position to one another .
Several double bonds that are separated by exactly one single bond are referred to as conjugated , those directly adjacent to one another as cumulated and, in the case of more than one single bond, as isolated until the next double bond .
Typical chemical reactions of molecules with a double bond
- C = C double bond ( alkenes ):
-
electrophilic addition to the double bond
- Another (electrophilic) molecule is deposited either after attack by E + (E + = electrophile) and ionic intermediate with breaking of the double bond on the molecule or in electrophiles with larger atoms (e.g. bromine ) with formation of a ring-shaped, ionic one Intermediate stage ("- onium ion", here also bromonium ion) with subsequent attack on the rear side of the remainder (in the example Br - ).
-
radical addition to the double bond
- a molecule whose bond can easily be split homolytically (into two radicals) (e.g. chlorine ) is split into two radicals under light (UV), which then attack the double bond.
-
electrophilic addition to the double bond
- C = O double bond ( carbonyl , ketone , aldehyde , carboxylic acid , carboxylic acid ester ):
- Reduction of the C = O bond to alcohols with reducing reagents such as hydrogen or Grignard reactions .
- Oxidation of the C = O bond (only aldehyde) to a carboxylic acid.
- Passerini reaction .
- C = N double bond
- Addition of hydrocyanic acid with formation of α-aminonitriles.
- Ugi reaction
- pericyclic reactions
For further reactions, see also name reactions , cumulative double bond .
Detection of C = C double bonds
A fairly unspecific detection of organic compounds with C = C double bonds, ie of alkenes , is possible using bromine water . If an alkene is present, the bromine water , which has been colored yellow by Br 2, is decolorized with the sample after shaking. A bromoalkane is formed from the alkene via an addition reaction . For details see alkene detection .
See also
- Double bond rule
- Double bond equivalent
- Cis - trans isomerism [( EZ ) isomerism]
- π-π interaction
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 54, ISBN 3-342-00280-8 .



