Polyoxymethylene
| General structures of polyoxymethylenes |
| Constitutional repeating unit (oxymethylene group) of POM-H. Formal or real monomer for synthesis is formaldehyde . |
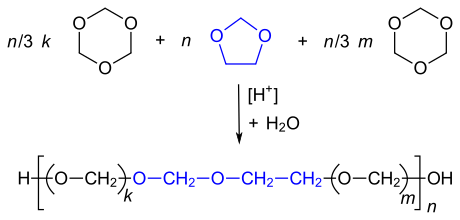
| Constitutional repeat units of POM-C, where m is much smaller than k . Comonomers are either dioxolane or ethylene oxide , which are only added in small amounts during the synthesis. |
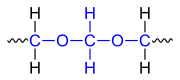
| Structural element of the unbranched acetal group, which is marked in blue . They are available from POM-H and POM-C and justify the alternative designation of these polymers as polyacetals. |
Polyoxymethylenes ( abbreviation POM, also polyacetals called) are high molecular weight thermoplastic polymers . The colorless, partially crystalline polymers are mainly used for the production of molded parts by injection molding. Because of their high rigidity , low coefficients of friction and good dimensional stability , polyoxymethylenes are used for the manufacture of precision parts. A defining structural element is an unbranched acetal group (~ C – O – CH 2 –O – C ~).
Consisting of formaldehyde produced homopolymer is referred to as POM-H and has the structure - (CH 2 -O-) n (oxymethylene groups). In addition to numerous acetal groups, the copolymers (abbreviation POM-C) also have - (CH 2 ) m –O– units with 2 or 4 methylene groups. They serve to thermally stabilize the polymer. POM-C has similar properties to POM-H.
history
In the 1920s, Hermann Staudinger and Werner Kern systematically investigated the polymerization of formaldehyde. However, it was not possible to produce stabilized polymers with a sufficiently high degree of polymerization. In the 1940s, DuPont researched the production of pure formaldehyde and succeeded in producing POM-H, which was patented in 1956. In the late 1950s, POM-H was produced and marketed under the trade name Delrin .
Celanese (United States) developed POM-C, which was marketed under the trade name Celcon in 1961 . In a joint venture with Hoechst (now Ticona Kelsterbach ), POM-C was manufactured in Germany and sold under the trade name Hostaform . In 1971 POM-C came onto the market under the name Ultraform ( BASF ).
properties
| properties | POM-H | POM-C |
| CAS number | 9002-81-7 | |
| density | 1.43 g / cm 3 | 1.41 g / cm 3 |
| Melting temperature | 182 ° C | 166 ° C |
| Glass transition temperature | −60 ° C | −60 ° C |
| Short-term, max. Application temperature | 150 ° C | 140 ° C |
| Long-term, max. Application temperature | 110 ° C | 100 ° C |
| Thermal expansion coefficient 23–100 ° C | 23 · 10 −5 · K −1 | 14 · 10 −5 · K −1 |
| Ball indentation hardness | 185 MPa | 165 MPa |
| Coefficient of sliding friction against steel | 0.34 | 0.32 |
| Surface resistance | 1.00 x 10 +14 Ω | 1.00 x 10 +14 Ω |
| Water absorption | 0.050% | 0.050% |
| Resistance to hot water / alkalis | inconsistent | conditionally resistant |
POM is characterized by high strength , hardness and stiffness in a wide temperature range. It retains its high toughness, has high abrasion resistance, a low coefficient of friction , high heat resistance , good sliding properties, good electrical and dielectric properties and low water absorption . The intrinsic color is opaque white because of the high crystallinity , but the material can be colored in all colors.
The crystallinity of POM-H reaches 80% and is slightly higher than that of POM-C with up to 75%. The crystallite melting point of POM-H is 175 ° C and of POM-C between 164 and 172 ° C. Because of the higher crystallite melting point, POM-H has a slightly better heat resistance. POM-C is somewhat more resistant to alkalis and hot water. In general, POM has a poor weather resistance. If the processing temperature is too high or if it is heated above 220 ° C, POM begins to thermally decompose. Among other things, formaldehyde is formed , which has a pungent and irritating odor.
Without special surface treatment, POM can only be bonded to a limited extent . The adhesion of adhesives (mostly epoxy resins ) can be improved by special pickling of the surface .
Manufacturing
A distinction is made between homopolymer and copolymer , which can be produced using different processes.
Homopolymer
| Monomers | |
 formaldehyde |
 Trioxane |
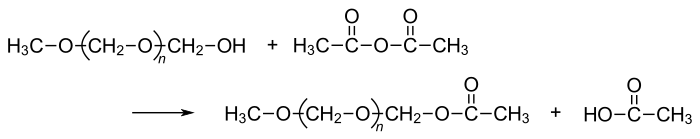
Polyoxymethylene homopolymer (POM-H) has the structure - (CH 2 –O–) n and is produced from formaldehyde or from trioxane . This is why POM-H is also known as polyformaldehyde or polytrioxane. Polyoxymethylene can be obtained by cationic ring-opening chain polymerization of trioxane. However, POM-H is mostly obtained by anionic chain polymerization of formaldehyde. In both cases the resulting macromolecules end up as less stable hemiacetals (R – CH 2 –O – CH 2 –OH). Under the influence of acid or thermal stress, this leads to depolymerization with the release of formaldehyde. The end groups are closed by esterification for stabilization .
Polyformaldehyde
Formaldehyde is produced in a suspension polymerization in cyclohexane as a solvent. In the first step, anhydrous formaldehyde is obtained. The comparatively short-chain paraformaldehyde is depolymerized to formaldehyde under heat :
The polymerization is initiated by a base such as sodium methoxide .
The growth takes place in an anionic chain polymerization .
Termination occurs due to traces of water or methanol.
The polymer is stabilized against thermal depolymerization by reaction with acetic anhydride .
Polytrioxane
Trioxane is obtained by trimerizing formaldehyde in an acidic aqueous solution. The cyclization takes place in a nucleophilic addition of the carbonyl-O to the carbonyl-C of a neighboring aldehyde molecule.
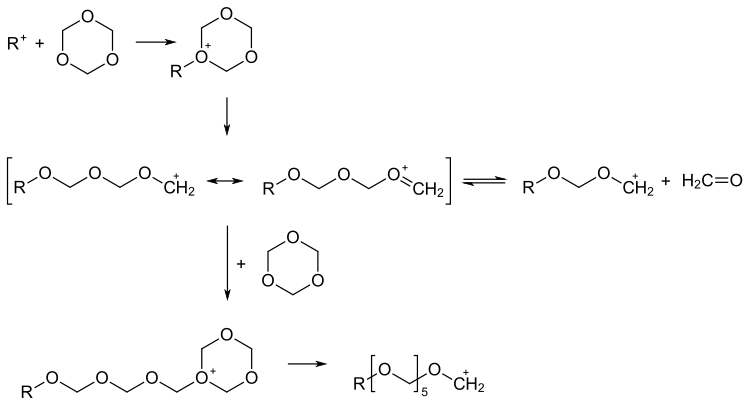
The polymerization of trioxane takes place as a cationic ring-opening chain polymerization . Strong protic acids such as sulfuric acid , trifluoroacetic acid or boron trifluoride BF 3 (also in the form of boron trifluoride diethyl etherate ) and salts such as iron (III) chloride (FeCl 3 ), tin (IV) chloride (SnCl 4 ) are used as catalysts .
The cationic initiator attaches itself to an oxygen atom of the trioxane and leads to the formation of an oxonium ion in the first step . In a start-up phase only formaldehyde and oligomers are formed. The system then strives for an equilibrium between formaldehyde and oligomers. Only then does the propagation to macromolecules begin.
Stabilization of the polymer against thermal depolymerization is also necessary here. The addition of other cyclic ethers, such as dioxolane , is obvious , which leads to a stable copolymer.
Copolymer
| Comonomers | ||
|
Oxirane |
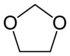 Dioxolane |
 1,3-dioxepane |
The copolymers (POM-C) are produced in a cationic ring-opening copolymerization of trioxane with oxirane , dioxolane or 1,3-dioxepane . The proportion of the comonomer is in the range from 2-4%. The comonomers are incorporated into the growing polymer in a randomly distributed manner.
For thermal stabilization of the macromolecules with unstable hemiacetals as the end group, formaldehyde is split off heterogeneously by hydrolysis with NH 3 at 100 ° C. or in a homogeneous melt at 170-220 ° C. until there is no hemiacetal as an end group.
processing
The material can be processed by injection molding at 180 to 220 ° C (POM-H) or 180 to 230 ° C (POM-C). It can also be processed by extrusion blow molding . POM semi-finished products are well suited for further machining.
use

- Mechanical engineering : gears , sliding and guide elements, housing parts , spring elements , chains , screws , nuts , fan wheels , pump parts , valve bodies
- Electrical engineering : insulators , bobbins , connectors , parts for electronic devices, e.g. B. Televisions and Phones
- Vehicle construction : steering column (including shift lever for lights, indicators), window lifters, door lock systems, joint shells
- Model construction : thin-walled, more highly stressed parts of model railways, such as bogies and handle bars. POM breaks less easily than ABS when subjected to stress , but is translucent in light colors and cannot be painted.
- Medicine : insulin pen
- Furniture construction : fittings , locks , handles , hinges or even curtain rolls
- Construction : Sleeves for point fixings in structural glass construction
- Packaging : aerosol cans , vehicle tanks, gas ampoules
- Sports : paintball accessories , especially bolts
- Clothing : zippers
- Music : Tortex - plectra for plucked instruments as a replacement for plectra made of tortoiseshell ; Delrin quills for harpsichords to replace raven feather quills ; some wind instruments, particularly Irish flutes and tin whistles
- Gastronomy : the brewing group in fully automatic coffee machines
- Physical chemistry : the sliding barrier of the film scale . The hydrophobic material is heavy enough to prevent the monolayer from slipping through.
- Lighters : housing
- E-cigarette : driptips
The use of POM for the production of consumer goods that come into contact with food is regulated in the Food, Consumer Goods and Feed Code. Only POM with a melt index of max. 50 g / 10 min (MFI 190 / 2.16) may be used. In addition, the maximum permissible proportions of catalysts and other substances required for production and processing are regulated. POM is not suitable for the storage and packaging of acidic products with a pH value below 2.5.
Market shares
Market leaders in the approx. 780,000 t POM market in 2006 were:
- Ticona approx. 25%
- DuPont approx. 20%
- Polyplastics approx. 20%
- Mitsubishi Gas Chemical approx. 10%
- KEP approx. 10%
- Others approx. 15%
Individual evidence
- ^ Wolfgang Kaiser, Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 389.
- ↑ a b Sigrid Lüftl, PM Visakh, Sarath Chandran: Polyoxymethylene Handbook: Structure, Properties, Applications and Their Nanocomposites , Wiley, New Jersey, 2014, p. 2f. (Limited preview)
- ↑ a b c d e f g h i j k KUNDERT AG plastics database, entry POM-H
- ↑ a b c d e f g h i j k KUNDERT AG plastics database, entry POM-C
- ↑ a b c d Wolfgang Kaiser, Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 384f.
- ↑ a b Bernd Tieke: Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 128.
- ↑ Gerd habenicht: Gluing: Basics, Technology, Applications , Springer-Verlag, Berlin, 2013, p. 616. (Restricted preview)
- ↑ Bernd Tieke: Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 113.
- ↑ Eberhard Breitmaier, Günther Jung: Organischen Chemie , 7th edition, Thieme, Stuttgart, 2012, p. 321.
- ↑ Olagoke Olabisi, Kolapo Adewale: Handbook of Thermoplastics , CRC-Press, Boca Raton, 2016, p. 268. (Restricted preview)
- ^ Wolfgang Kaiser: Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 386ff.
- ↑ Bernd Tieke: Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 116.
- ↑ Description by Duran Group .
- ^ Plastics in the food traffic Carl Heymanns Verlag KG, XXXIII. Acetal resins as of June 1, 2007.
- ↑ Polyoxymethylene (POM) , kunststoffe.de.


![{\ displaystyle \ mathrm {HO ({-} CH_ {2} {-} O) _ {\ mathit {n}} {-} H \ \ {\ xrightarrow [{}] {\ Delta}} \ \ {\ mathit {n}} \ H_ {2} C {=} O \ + \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/91837070991a5af2211213634509829d2a41c737)




![{\ displaystyle \ mathrm {R {-} O {-} CH_ {2} {-} O {-} CH_ {2} {-} CH_ {2} {-} O {-} CH_ {2} {-} O {-} CH_ {2} {-} OH \ \ {\ xrightarrow [{}] {\ Delta}} \ \ R {-} O {-} CH_ {2} {-} O {-} CH_ {2 } {-} CH_ {2} {-} O {-} H \ + \ 2 \ H_ {2} C {=} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ab91a9cd01f968d413596f5323a8233b3155f6e)