Photopolymer
A photopolymer is a polymer that changes its properties when it is irradiated with light from the UV - VIS range of the electromagnetic spectrum. These are structural changes, such as B. the photochemical curing of the material through crosslinking . The example below shows a mixture of monomers , oligomers and photoinitiators that converts into a hardened material during photopolymerization .
There is a wide variety of technologically useful applications for photopolymers, for example there are paints, dental fillings and lacquers that are based on light-dependent polymerization for curing. In some cases, fractures can be repaired photochemically in seconds, which would take half an hour or more with thermal heating. Repair materials are often used in medical technology, in printing and in photoresist technology .
Photopolymers are among the functional polymers .
properties
Changes in the structural and chemical properties can be brought about internally by chromophores that the polymer unit already has, or externally by adding photosensitizers . A typical photopolymer consists of a mixture of multifunctional monomers and oligomers that polymerize, and for this purpose a variety of such molecules have been developed which polymerize in the presence of light (or in the presence of another free radical initiator ). Photopolymers are subject to a process of photo-curing or curing, whereby the oligomers that are exposed to light cross-link or branch. The result is a network of polymers. One advantage of photohardening is that it can be used selectively when using lasers as the light source . Often, however, photoinitiators are required to start the polymerization. These are compounds which, when exposed to light, break down into reactive molecules that act as radicals to initiate the polymerization of the individual oligomers. An example of a mixture that crosslinks when exposed is shown below. It consists of the monomer styrene and the oligomer acrylate .
Most photopolymer systems are hardened by UV light because it is more energetic than visible light, but dye-based photoinitiator systems have already been developed for visible light, which have the advantage that they are easier and safer to handle. UV curing has gained considerably in importance in industrial processes over the past few decades. Many thermal and solvent-based technologies have been replaced by photopolymerization. The advantage of photochemistry is a high degree of polymerisation and better environmental compatibility compared to the use of volatile organic solvents. There are two different mechanisms for starting photopolymerization: radical and ionic. The general reaction is to add a small amount of photoinitiator to the polymer, followed by selective exposure to light, resulting in a highly crosslinked product. For most of such reactions it is not necessary to use a solvent, so that reactions of the initiator with the solvent and thus impurities are prevented, in addition, the overall costs are reduced.
Mechanisms
Ionic mechanism
In the ionic curing process, a photoinitiator is used to activate a functional group on an oligomer so that it can crosslink. Typically, photopolymerization is a selective process and it is critical that it only take place where it is intended. This solvent-free reaction is carried out with the addition of an anionic or cationic photoinitiator only when irradiated with light. Monomers used for cationic polymerization are: styrene , vinyl ethers, N- vinyl carbazoles , lactones , lactams, cyclic ethers , cyclic acetals and cyclic siloxanes . The majority of ionic photoinitiators are cations, the anions are less explored. There are a number of cationic initiators: onium salts , organometallic compounds and pyridinium salts . As mentioned above, one of the disadvantages of photoinitiators is that they tend to absorb light from the UV range. Photosensitizers or chromophores that absorb light of a much shorter wavelength can be used to photochemically excite the photoinitiator through energy transfer. Another variation of this type of reaction is radical-assisted cationic polymerization. In this case a free radical reacts with the photoinitiator to start the polymerization. Most of the compounds used industrially are epoxides , oxetanes and vinyl ethers. One of the advantages of cationic photopolymerization is that the polymerization is no longer sensitive to oxygen and therefore no inert gas atmosphere is required.
Cationic photoinitiators
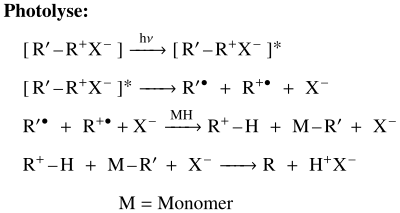
The proposed mechanism for cationic polymerization begins with the excitation of the photoinitiator. Both the homolytic cleavage and the dissociation of a counter-anion take place at the same time, so that a radical cation (R + ), an aryl radical (R´) and an anion (X - ) are generated. The removal of a Lewis acid (a hydrogen in the picture above) from a radical cation (R) produces a weakly bound proton and a free radical. The acid is later deprotonated by the anion in the solution by forming a Lewis acid with the starting anion (X - ) as the counterion. It is believed that the acid proton ultimately causes the polymerization.
Onium salts
Since their discovery in 1970, aryl onium compounds , especially iodonium and sulfonium salts, have gained in importance and have found application in industrial processes. Other less common onium salts that are not mentioned here are, for example, ammonium and phosphonium salts.
| Onium salts |
|---|
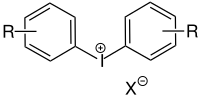
Iodonium salt |

Sulfonium salt |
| For the meaning of R and X: See accompanying text. |
The typical onium compound used as a photoinitiator contains two or three aryl groups for iodonium and for sulfonium, respectively. Onium salts generally absorb light in the UV range from 225 to 300 nm. A decisive feature for the suitability of the onium salt as a photoinitiator is that the central anion is not nucleophilic. The acid formed in the first reaction step is the radical initiator for the polymerization. A possible final reaction of the chain reaction would be if the central ion of the onium compound reacts as a nucleophile instead of a functional group of the oligomer.
Organometallic compounds
Although less common, transition metal complexes can also act as cationic photoinitiators. In general, the mechanism is simpler than that described for onium salts above. Most photoinitiators consist of a metal salt with a non-nucleophilic central anion. For example, ferrocenes are very important for commercial applications. The absorption band for ferrocene complexes lies in the visible range of the spectrum. Upon exposure, the metal center loses a ligand, which is replaced by a functional group, causing polymerization to begin. A disadvantage of this method is its sensitivity to oxygen. There are some organometallic photoinitiators that react according to similar mechanisms. In anionic polymerization, the photochemical excitation of the central atom is followed by bond cleavage or electron transfer, which generates the active anion, the photoinitiator.
Pyridine salts
In general, those pyridines are used as photoinitiators that are N-substituted and carry a positive charge on the nitrogen atom . The central ion is a non-nucleophilic anion. Upon irradiation, a homolytic bond cleavage takes place, which generates a pyridine radical cation and a free radical. Most of the time, the prydin radical removes a hydrogen atom from the oligomer. The free radical generated in this way is captured by a radical in the solution. The result is a strong pyridic acid which, as a free radical initiator, initiates the polymerization.
Radical mechanism
Before the radical mechanism of polymerization was clarified, it was observed that certain monomers polymerize on exposure to light. It was then found that many compounds can be cleaved by light and used them as photoinitiators (starters) in polymer production. In the radical mechanism of UV curing systems, light is absorbed by the photoinitiator, which generates free radicals that lead to the crosslinking of a mixture of functionalized monomers and oligomers , creating a polymer film or photoresist . Photo-curing materials based on a radical mechanism run in a chain reaction that consists of three basic steps:
- Start reaction (initiation)
- Chain growth (propagation)
- Chain termination
These three steps are outlined in the following scheme, where R • is the radical that is generated by light during the initiation reaction, and M is the monomer. The active monomer is formed in a radical reaction, during which it grows into a radical polymer chain. In UV-curing materials, the growth step means that the chain radicals react with reactive double bonds of the oligomers. The final reaction comes about when two chain radicals meet (combination) or when a hydrogen atom is transferred to another radical chain ( disproportionation ), so that two polymer chains are created.
Most of the mixtures that harden through chain growth contain a mixture of momomers and oligomers with a molar mass of 500 to 3000 u. In general, monomers with a higher functionality have a higher crosslink density in the finished polymer film. Typically, the monomers and oligomers do not absorb enough energy from the light sources used or not in the appropriate wavelength range, which is why photoinitiators are added.
Photo initiators
There are two types of photoinitiators that generate free radicals: a two-component system, where the radical is generated by separating a hydrogen atom from a donor compound, and a one-component system, where two radicals are generated by bond cleavage:
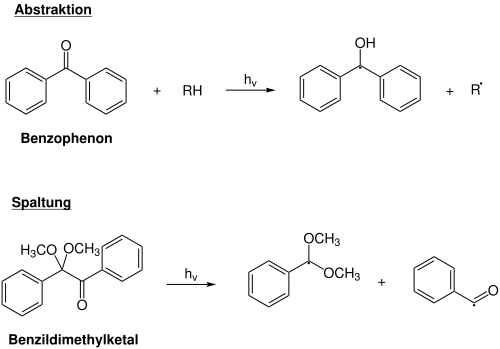
Benzophenone , xanthone and quinone are examples of the first type with aliphatic amines as the donor component. The generated radical R • becomes the radical initiator in the polymerization process.
Benzoyl ether , acetophenone , benzoyloxime and acylphosphine are examples of bond-cleaving photoinitiators. The bond cleavage typically results in the generation of two radicals during the absorption of light , and both radicals can start the polymerization. This type of free-radical initiator does not require any further additives, such as aliphatic amines. This is advantageous since the chain length and crosslink density of the resulting polymer film are not reduced by further additives.
Oligomers and monomers
The properties of the photo-cured material, such as flexibility, adhesion and chemical resistance, are based on the oligomers present in the photo-cured mixture. Oligomers are typically epoxies , polyurethanes , polyethers or polyesters , all of which components cause the specific material properties of the resulting product. In the example shown, an epoxy oligomer is used, functionalized by an acrylate. Acyl epoxides are useful paints for metals. Acyluretane oligomers are typically flexible and are ideal coatings for floors, printing plates and packaging materials. Acrylic polyethers and polyesters produce films that are sparingly soluble in acids, but are not resistant to UV light and therefore cannot be used as UV-curing material. Often the reaction mixtures are made from different oligomers in order to create the right material properties.
The monomers for UV-curing substances control the curing speed, the crosslinking density, the surface properties of the polymer film and the viscosity of the granulate. Examples of monomers are styrene , N- vinylpyrrolidone and acrylate . Styrene is an inexpensive monomer that cures quickly, N-vinylpyrrolidone has a high flexibility and is not toxic, and acrylates are very reactive, cure quickly and are versatile (with monomers from mono- to tetrafunctional.) The monomers make, like the oligomers , the desired properties of the end product.
Applications
Photopolymerization is a widely used technology that is used for imaging and biomedical applications.
Medical use
Dentistry is a market where radical polymerization is widely used as an adhesive or as a material for fillings and protective varnishes. These fillings are based on camphorquinone as an initiator, a matrix of methacrylate oligomers and an inorganic filler such as silicon dioxide . UV curable adhesives are also used in the production of catheters, hearing aids, breathing masks, medical filters and sensors for blood analysis . Photopolymers have also been used for drug delivery , tissue engineering, and cell capsules. Photopolymerization processes for these applications are carried out in vivo or ex vivo. In vivo photopolymerization would have the advantage of being used for production and implantation in minimally invasive procedures. Ex vivo photopolymerization would enable the production of complex matrices and their versatile use. Although photopolymers offer a wide variety of new biomedical applications, biocompatibility has yet to be tested and developed.
3D imaging
Stereolithography , digital imaging, and 3D inkjet printing are just a few 3D techniques that use photopolymers. 3D imaging is typically performed using computer-aided technologies such as: B. Software that converts a 3D image into a three-dimensional 3D object. 3D imaging is usually done with software that creates a 3D image that is then converted into a plastic three-dimensional body. The image is cut into slices, each slice being copied while UV curing the liquid polymer, thus converting the image into a solid object. Photopolymers for 3D imaging are designed to have a small decrease in volume upon polymerization to prevent shrinkage of the solid object. The monomers typically used for 3D imaging are multifunctional acrylates and methacrylates combined with a non-polymerizing compound to prevent volume shrinkage. A suitably composed mixture of epoxides with cationic photoinitiators is used to an increasing extent, since the volume decrease during the ring opening of the epoxide is significantly less than that of the acrylates and methacrylates. Free-radical and cationic polymerizing mixtures containing epoxies and acrylate monomers are also used, whereby one gains high polymerization rates through the acrylate component and very good mechanical properties through the epoxy matrix.
Photoresists
Photoresists are photoresists or oligomers that are applied to a surface and are manufactured in such a way that they change their properties after exposure. This change means the photo-curing of a liquid oligomer to an insoluble network structure as a photoresist (negative paint) or, conversely, the decomposition of a solid polymer into liquid products (positive paint). Both have many uses in the design and production of microchips. The ability to make a resist using a light source led to its application in photolithography .
Negative resists
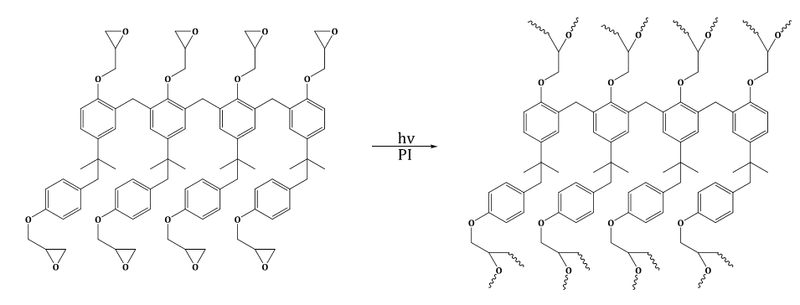
As mentioned above, negative resists are photopolymers that turn into insoluble paints when exposed to radiation. They have a wide range of applications, particularly in the design and manufacture of electronic microchips. The presence of functional groups on the polymers used is characteristic of the photoresists . The polymer irradiated with UV light results in a chemically stable polymer network in the presence of a photoinitiator . A common functional group for a photoresist is an epoxy .
This is an example of intramolecular polymerization, in which a matrix is formed from a branched material.
Positive resists
As already mentioned, positive resists change their chemical structure when exposed to radiation and become liquid (or better soluble). The structural change is based on a bond break within the polymer network. The polymer decomposed by the irradiation can be washed away with a developer solution; the unexposed polymer remains. This technique allows the production of very fine stencils for microelectronics . For these applications, a positive resist needs polymers with easily cleavable branches and a photoinitiator that opens bonds in the polymer. A polymer that liquefies or becomes a soluble product upon irradiation is used as a positive photoresist. Polymers whose functional groups are hydrolyzed by a photochemically generated catalyst are, for example, polycarbonates and polyesters.
Fine print
Photopolymers can be used to make printing plates that can then be used to print on paper, such as metallic printing plates. This technique is often used in newspaper printing to make letters appear three-dimensional. The design can be created on the computer without having to scratch it in metal. It is often used for business cards.
Individual evidence
- ↑ a b c d e f g Elsa Reichmanis, James Crivello: Photopolymer Materials and Processes for Advanced Technologies . In: Chem. Mater. tape 26 , 2014, p. 533-548 .
- ^ Roger Phillips: Photopolymerization . In: Journal of Photopolymerization . tape 25 , 1984, pp. 79-82 , doi : 10.1016 / 0047-2670 (84) 85016-9 .
- ↑ Jeff Burton: A Primer on UV-Curable Inkjet Inks. Specialty Graphic Imaging Association, accessed August 7, 2017 .
- ↑ a b c d e f g A. Ravve: Light-Associated Reactions of Synthetic Polymers . Springer Science + Business Media, New York 2006, ISBN 0-387-31803-8 .
- ↑ a b c d e f Jean Pierre Fouassier: Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency . Wiley-VCH Verlag, Weinheim 2012, ISBN 978-3-527-64824-5 .
- ^ Radiation Chemistry in EB and UV Light Cured Inks. Paint & Coatings Industry, accessed August 7, 2017 .
- ↑ a b J. P. Fouassier, X.Allonas, D. Burget: Photopolyermziation reactions under visible lights: principle, mechanisms and examples of applications . In: Progress in Organic Coatings . tape 47 , 2003, p. 16-36 , doi : 10.1016 / S0300-9440 (03) 00011-0 .
- ^ A b J. Crivello, E. Reichmanis: Photopolymer Materials and Processes for Advanced Technologies . In: Chemistry of Materials . tape 26 , 2014, p. 533-548 , doi : 10.1021 / cm402262g .
- ↑ Viktor Zhdankin: hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds . John Wiley & Sons, 2013, pp. 427 .
- ↑ Jean Fouassier: Photoinitiators for Polymer Synthesis: Scope, Reactivity, and Efficiency . John Wiley & Sons, 2012, p. 293 .
- ^ K. Meier: Proceedings of the RadCure Europe . Basle Technical Paper, 1985.
- ↑ Eiji Takahashi, Fumio Sanda, Takeshi Endo: Novel pyridinium salts as cationic thermal and photoinitiators and their photosensitization properties . In: Journal of Polymer Science Part A: Polymer Chemistry . tape 40 , no. 8 , 2002, p. 1037 , doi : 10.1002 / pola.10186 .
- ^ A b c Charles Hoyle: Radiation Curing of Polymeric Materials . At the. Chem. Soc., Washington, DC 1990, pp. 1-15 .
- ↑ Biancamaria Baroli: Photopolymerization of biomaterials . In: J. Chem. Technol. Biotechnol. tape 81 , 2006, p. 491-499 , doi : 10.1002 / jctb.1468 .
- ↑ SU-8 Photosensitive Epoxy. (No longer available online.) Archived from the original on May 30, 2012 ; accessed in 2014 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Harry Allcock: Introduction to Materials Chemistry . Wiley and Sons, 2008, ISBN 978-0-470-29333-1 , pp. 248-258 .
- ^ Larry Thompson: Polymers for Microelectronics . American Chemical Society, 1993.
- ↑ What is a “faux-emboss”? Dolce Press, accessed September 24, 2015 .
- ↑ Letterpress polymer plate service. Old City Press, accessed September 24, 2015 .
- ↑ What is letterpress? Baltimore Print Studios, accessed September 24, 2015 .