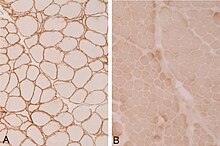
Belt dystrophy
| Classification according to ICD-10 | |
|---|---|
| G71.0 | Muscular dystrophy - pelvic or shoulder girdle shape |
| ICD-10 online (WHO version 2019) | |
The term limb- (Engl. Limb-girdle muscular dystrophy, LGMD ) is a group of hereditary muscle diseases ( myopathies ), the common feature of paralysis of the muscles of the shoulder and pelvic girdle are. The shoulder and pelvic girdles are collectively referred to in medicine as the limb girdle . In addition to muscle paralysis, the limb girdle dystrophies meet the criteria of muscular dystrophy . Muscular dystrophy is characterized by remodeling processes in the muscle tissue. The diseases in this group are genetically and clinically heterogeneous. They are caused by different gene mutations and the same gene mutations can produce a very variable clinical picture. The onset of the disease ranges from toddler to advanced adulthood and both mild and severe courses are possible. Girdle dystrophies are rare diseases. Estimates of the incidence (prevalence) of all limb girdle dystrophies range from 1: 14,500 to 1: 123,000.
causes
Girdle dystrophies are caused by genetic defects on different chromosomes . These lead to structural deviation, deficiency or complete absence of various muscle proteins , which leads to chronic damage to the muscle fibers that are frequently used. The main symptom is the increasing weakness and the visible shrinkage of the affected muscle areas.
Classification
The classification that is common today is based on the classification of the genetic mutations or the modified proteins. After the inheritance two groups can be distinguished: the autosomal - dominant inherited limb-girdle muscular dystrophy, LGMD1 and the autosomal recessive inherited limb-girdle muscular dystrophy, LGMD2 .
Autosomal dominant forms of limb girdle dystrophies
The autosomal dominant hereditary girdle dystrophies are relatively rare and account for about 10% of all girdle dystrophies. The diseases usually manifest themselves in adulthood ( adult-onset ) and the clinical course is often less severe compared to the autosomal recessive inherited limb girdle dystrophies.
| Type | Locate | gene | Gene product | Age of manifestation | Guiding symptoms | forecast |
|---|---|---|---|---|---|---|
| LGMD1A | 5q22-q34 | MYOT | Myotilin | 18-35 | Weakness of the leg muscles near the hips; years later, arm muscles are affected; Weakness of the facial and throat muscles in 20%, speech disorders ( dysarthria ) in 25% | Wheelchair dependency around 20 years after onset, mostly normal life expectancy |
| LGMD1B | 1q22 | LMNA | Lamin-A / C | 4-30 | Weaknesses in the hip and thigh muscles near the hips, in two-thirds of cases conduction disorders of the heart and possibly weak heart muscle with enlargement of the heart (dilated cardiomyopathy ) | Lifelong ability to walk, but risk of sudden cardiac death by 50–60 years |
| LGMD1C | 3p25.3 | CAV3 | Caveolin-3 | 2-20-70 | Muscle weakness close to the trunk, calf cramps, calf pseudohypertrophy | likely lifelong walking ability and normal life expectancy |
| LGMD1D | 6q23 | OF | Desmin | 20-25, rarely under 20 | Arrhythmia and cardiomyopathy , later additional muscle weaknesses near the trunk | Sudden death from AV block , ventricular tachycardia, or cardiomyopathy possible |
| LGMD1E | 7q | not known | not known | 10-30 | Leg muscle weakness near the hip | |
| LGMD1F | 7q32.1 | TNPO3 | Transportin 3 | under 1–58 | Pelvic girdle, shoulder girdle muscle weakness | |
| LGMD1G | 4q21 | HNRNPDL | D-like heterogeneous nuclear ribonucleoprotein | 30-47 | Pelvic and shoulder girdle weakness, flexion contractures of the toes and fingers | |
| LGMD1H | 3p25.1-p23 | not known | not known |
Autosomal recessive forms of limb girdle dystrophies
| Type | Locate | gene | Gene product | frequency | Age of manifestation | Guiding symptoms | forecast |
|---|---|---|---|---|---|---|---|
| LGMD2A | 15q15.1 | CAPN3 | Calpain-3 | About 10% of all LGMD2, founder mutations : Amish , La Réunion , Basques , Turks | 3-10-30 | Weakness of the hip and thigh muscles, including the trunk muscles, shoulder muscles often 2–5 years later, often also contractures of the spine , ankles, elbows and hands | rapid or slow course, depending on death around the age of 20 possible, otherwise in the 8th decade of life, early onset does not automatically mean rapid progression |
| LGMD2B | 2p13.2 | DYSF | Dysferlin | About 5% of all LGMD2 | 13-22-35 | Weakness especially of the posterior hip and thigh muscles near the hip, 25% calf pseudohypertrophy, 2–10 years later shoulder muscle weakness | rapid or slow course, about 30% ability to walk up to 26–54 years, otherwise up to the 8th decade |
| LGMD2C | 13q12.12 | SGCG | Gamma sarcoglycan | 3-12 | Muscle weakness in the pelvic girdle earlier than in the shoulder girdle, early calf pseudohypertrophy, later facial muscles may be affected | Walking ability 25% 10–15 years, 50% 15–20 years, 25% over 20 years, death possible in the second decade of life | |
| LGMD2D | 17q21.33 | SGCA | Alpha sarcoglycan | 1-16, later | Increasing muscle weakness in the pelvic girdle and thighs with delayed starting to walk or an unsteady gait similar to Duchenne muscular dystrophy | rapid or slow course, ability to walk up to 16-30 years or later, premature death possible if the course progresses rapidly | |
| LGMD2E | 4q12 | SGCB | Beta sarcoglycan | 3-12 | Pelvic girdle more affected than shoulder girdle, calf pseudohypertrophy | rapid or slow course, ability to walk 9–14 years or up to 38 years, death in the 2nd – 3rd Decade of life possible | |
| LGMD2F | 5q33-q34 | SGCD | Delta sarcoglycan | 4-10 | Increasing muscle weakness in the pelvic girdle and thighs with delayed starting to walk or an unsteady gait similar to Duchenne muscular dystrophy , facial muscles possibly affected, calf hypertrophy | Rapid progression with the ability to walk 9–16 years, death possible at the end of the first or in the second decade of life | |
| LGMD2G | 7q12 | TCAP | Telethonin | About 3% of all LGMD2 | 9-15 | In addition to hip and thigh muscle weakness, lower leg muscles and arm / shoulder muscles are also affected | long walking ability up to 18-25 years after beginning |
| LGMD2H | 9q33.1 | TRIM32 | E3 ubiquitin protein ligase TRIM32 | 1st - 3rd decade | Pelvic girdle more affected than shoulder girdle, possibly neck or back pain | Walking difficulties at 37–46 years of age, wheelchair dependency in the seventh decade of life | |
| LGMD2I | 19q13.32 | FKRP | Fukutin Associated Protein (FKRP) | About 6% of all LGMD2 | 1st – 4th decade | Initially weakness of the hip and thigh muscles, later the shoulder and upper arm muscles, calf pseudohypertrophy, muscle pain during exercise, muscle fiber breakdown ( rhabdomyolysis ) | very different course, walking ability mostly up to 30 years |
| LGMD2J | 2q31.2 | TTN | Titin | 1st - 3rd decade | Muscle weakness and atrophy of the hip and thigh muscles near the hips | Loss of walking in the 3rd – 5th Decade of life | |
| LGMD2K | 9q34.13 | POMT1 | Protein O-mannosyl transferase 1 | ||||
| LGMD2L | 11p14.3 | ANO5 | Anoctamine-5 | About 25% of LGMD2 in the UK , Founding Mutations : Northern Europeans | |||
| LGMD2M | 9q31.2 | FKTN | Fukutin | 6 patients | |||
| LGMD2N | 14q24.3 | POMT2 | Protein O-mannosyltransferase 2 | 2 patients | |||
| LGMD2O | 1p34.1 | POMGNT1 | Protein-O-mannose-beta-1,2-N-acetylglucosaminyltransferase 1 | 2 independent patients (1 Irish girl, 1 Belgian boy) | |||
| LGMD2P | 3p21.31 | DAG1 | Dystroglycan | 1 Turkish patient, homozygous mutation | early childhood | Belt dystrophy with mental retardation | At 16, walking a few meters, IQ 50 |
| LGMD2Q | 8q24.3 | PLEC1 | Plectine | 4 members of a Turkish family | |||
| LGMD2R | 2q35 | OF | Desmin | 2 Turkish clans | |||
| LGMD2S | 4q35.1 | TRAPPC11 | TRAPPC11 | 3 Syrian families, 5 Hutterites | |||
| LGMD2T | 3p21.31 | GMPPB | Beta GDP mannose phosphorylase | 3 independent patients | |||
| LGMD2U | 7p21.2 | ISPD | Isoprenoid synthase domain-containing protein | ||||
| LGMD2V | 17q25.3 | ATM | Lysosomal Alpha-Glucosidase | ||||
| LGMD2W | 3p21.31 | LIMS2 | LIM and senescent cell antigen-like-containing domain protein 2 | Childhood | Limb girdle dystrophy with pseudohypertrophy of the calves and macroglossia , impaired left ventricular function from the 3rd decade | ||
| LGMD2X | 6q21 | BVES | Blood vessel epicardial substance (Popeye protein 1) | Adulthood |
diagnosis
The derivation of the electrical muscle activity ( electromyography , EMG) provides unspecific evidence of chronic muscle damage ( myopathy ). Imaging methods such as computed tomography (CT) or magnetic resonance tomography (MRT) can show particularly affected muscle groups. A significantly increased creatine kinase is often noticeable in the laboratory . If clinical symptoms are suspected, immunohistochemical or molecular genetic examinations of a muscle biopsy usually help to confirm the diagnosis.
therapy
An established drug therapy is not known for any of the limb girdle dystrophies. Symptomatic treatment focuses on physical therapy measures to maintain muscle strength, to train everyday movements and to prevent contractures. However, maximal strength training is considered unfavorable. Appropriate aids in the form of splints ( orthoses ), walking sticks , walkers or a wheelchair are also important . In the case of misalignments of the feet or the spine, surgical treatment measures can also be considered to restore the ability to walk. If there is an impairment of speech motor skills ( dysarthria ), speech therapy is necessary.
After operations or other circumstances of restricted mobility, early mobilization is important, as prolonged immobilization of patients with limb girdle dystrophy often leads to a loss of the ability to walk. Physiotherapy should be started the day after surgery and carried out consistently.
If the heart is involved, therapy for conduction disorders and other heart diseases may be possible.
Medical history
Most of the time, the term limb girdle dystrophies is traced back to a work by John Nicholas Walton and Frederick John Natrass from 1954. The first descriptions of limb girdle dystrophies go back to the work of Ernst von Leyden and Paul Julius Möbius in 1876 and 1879, respectively.
literature
- V. Nigro, M. Savarese: Genetic basis of limb-girdle muscular dystrophies: the 2014 update. In: Acta myologica: myopathies and cardiomyopathies: official journal of the Mediterranean Society of Myology / edited by the Gaetano Conte Academy for the study of striated muscle diseases. Volume 33, Number 1, May 2014, pp. 1-12, ISSN 1128-2460 . PMID 24843229 . PMC 4021627 (free full text).
- E. Pegoraro, EP Hoffman: Limb-Girdle Muscular Dystrophy Overview. GeneReviews [Internet]. University of Washington, Seattle, June 8, 2000, [updated August 30, 2012]. PMID 20301582 .
- L. Broglio, M. Tentorio et al .: Limb-girdle muscular dystrophy-associated protein diseases. In: The neurologist. Volume 16, Number 6, November 2010, pp. 340-352, ISSN 1074-7931 . doi: 10.1097 / NRL.0b013e3181d35b39 . PMID 21150381 . (Review).
- CT Rocha, EP Hoffman: Limb-girdle and congenital muscular dystrophies: current diagnostics, management, and emerging technologies. In: Current neurology and neuroscience reports. Volume 10, Number 4, July 2010, pp. 267-276, ISSN 1534-6293 . doi: 10.1007 / s11910-010-0119-1 . PMID 20467841 . (Review).
- K. Bushby: Diagnosis and management of the limb girdle muscular dystrophies. In: Practical neurology. Volume 9, Number 6, December 2009, pp. 314-323, ISSN 1474-7766 . doi: 10.1136 / jnnp.2009.193938 . PMID 19923111 . (Review).
- A. Ferbert , W. Kress: Clinic and genetics of the limb girdle dystrophies. In: Medical Genetics. Volume 21, Number 3, 2009, pp. 332–336, doi: 10.1007 / s11825-009-0171-x
- M. Guglieri, K. Bushby: How to go about diagnosing and managing the limb-girdle muscular dystrophies. In: Neurology India. Volume 56, Number 3, 2008 Jul-Sep., Pp. 271-280, ISSN 0028-3886 . PMID 18974553 . (Review).
- M. Guglieri, V. Straub et al .: Limb-girdle muscular dystrophies. In: Current Opinion in Neurology . Volume 21, Number 5, October 2008, pp. 576-584, ISSN 1350-7540 . doi: 10.1097 / WCO.0b013e32830efdc2 . PMID 18769252 . (Review).
- F. Norwood, M. de Visser et al .: EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. In: European journal of neurology. Volume 14, Number 12, December 2007, pp. 1305-1312, ISSN 1468-1331 . doi: 10.1111 / j.1468-1331.2007.01979.x . PMID 18028188 . (Review).
- V. Straub, K. Bushby: The childhood limb-girdle muscular dystrophies. In: Seminars in pediatric neurology. Volume 13, Number 2, June 2006, pp. 104-114, ISSN 1071-9091 . doi: 10.1016 / j.spen.2006.06.006 . PMID 17027860 . (Review).
- D. Fischer: Clinical and imaging differential diagnosis of limb girdle dystrophies. In: Klin Neurophysiol. Volume 37, No. 3, 2006, pp. 180-188, doi: 10.1055 / s-2006-940133 .
- J. Finsterer: Clinic and genetics of the limb girdle dystrophies. In: The neurologist. 2004, Volume 75, Number 12, pp. 1153-1166, doi: 10.1007 / s00115-004-1769-5 .
- J. Sarparanta et al: Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. In: Nat Genet. Volume 44, No. 4, Feb 26, 2012, pp. 450-455, S1-2. doi: 10.1038 / ng.1103 .
Web links
- Center for Neuromuscular Diseases at Washington University in St. Louis: Limb-Girdle Muscular Dystrophy (Lgmd) Syndromes2 (detailed information, histological images)
Individual evidence
- ↑ a b c d e f E. Pegoraro, EP Hoffman: Limb-Girdle Muscular Dystrophy Overview. GeneReviews [Internet]. University of Washington, Seattle, June 8, 2000, updated August 30, 2012. PMID 20301582. "
- ↑ A. Ferbert, W. Kress: Clinic and genetics of the limb girdle dystrophies. In: Medical Genetics . Volume 21, Number 3, 2009, pp. 332-336.
- ↑ a b c d e V. Nigro, M. Savarese: Genetic basis of limb-girdle muscular dystrophies: the 2014 update. In: Acta myologica: myopathies and cardiomyopathies: official journal of the Mediterranean Society of Myology / edited by the Gaetano Conte Academy for the study of striated muscle diseases. Volume 33, Number 1, May 2014, pp. 1-12, ISSN 1128-2460 . PMID 24843229 . PMC 4021627 (free full text).
- ↑ LGMD1A. In: Online Mendelian Inheritance in Man . (English), accessed September 20, 2013.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at U. Schara , W. Mortier: Neuromuscular Diseases (NME). Part 2: Muscular Dystrophies (MD). In: Monthly Pediatrics. Volume 151, 2003, pp. 1321-1341.
- ↑ a b LGMD1C. In: Online Mendelian Inheritance in Man . (English), accessed September 20, 2013.
- ↑ LGMD1D. In: Online Mendelian Inheritance in Man . (English), accessed September 20, 2013.
- ↑ LGMD1E. In: Online Mendelian Inheritance in Man . (English), accessed September 20, 2013.
- ↑ LGMD1F. In: Online Mendelian Inheritance in Man . (English), accessed September 20, 2013.
- ↑ MJ Melià, A. Kubota et al .: Limb-girdle muscular dystrophy 1F is caused by a microdeletion in the transportin 3 gene. In: Brain: a journal of neurology. Volume 136, Pt 5, May 2013, pp. 1508-1517, ISSN 1460-2156 . doi: 10.1093 / brain / awt074 . PMID 23543484 . PMC 3634201 (free full text).
- ↑ a b LGMD1G. In: Online Mendelian Inheritance in Man . (English), accessed June 3, 2014.
- ↑ a b N. M. Vieira, MS Naslavsky et al .: A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). In: Human Molecular Genetics . [electronic publication before printing] March 2014, ISSN 1460-2083 . doi: 10.1093 / hmg / ddu127 . PMID 24647604 .
- ↑ LGMD1H. In: Online Mendelian Inheritance in Man . (English), accessed June 3, 2014.
- ↑ LGMD2A. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2B. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2C. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2D. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2E. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2F. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2G. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2H. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2I. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2J. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2K. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ LGMD2L. In: Online Mendelian Inheritance in Man . (English), accessed November 13, 2011.
- ↑ a b LGMD2M. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b LGMD2N. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b LGMD2O. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b c LGMD2P. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b Y. Hara, B. Balci-Hayta et al .: A dystroglycan mutation associated with limb-girdle muscular dystrophy. In: The New England Journal of Medicine . Volume 364, Number 10, March 2011, pp. 939-946, ISSN 1533-4406 . doi: 10.1056 / NEJMoa1006939 . PMID 21388311 . PMC 3071687 (free full text).
- ↑ a b LGMD2Q. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b LGMD2R. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b LGMD2S. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ a b LGMD2T. In: Online Mendelian Inheritance in Man . (English), accessed October 12, 2013.
- ↑ LGMD2X. In: Online Mendelian Inheritance in Man . (English), accessed July 9, 2016.
- ↑ A. Ferbert, W. Kress: Clinic and genetics of limb-girdle muscular dystrophy. In: Medical Genetics. Volume 21, Number 3, 2009, p. 337, doi: 10.1007 / s11825-009-0171-x
- ↑ a b A. Ferbert, W. Kress: Clinic and genetics of limb-girdle muscular dystrophy. In: Medical Genetics. Volume 21, Number 3, 2009, p. 336, doi: 10.1007 / s11825-009-0171-x
- ↑ JN Walton, FJ Natrass: On the classification, natural history and treatment of the myopathies. In: Brain. 77, 1954, pp. 169-231, doi: 10.1093 / brain / 77.2.169 .
- ↑ Hereditary forms of progressive muscular atrophy; Lipomatous muscle hypertrophy. In: Clinic of Spinal Cord Diseases. Vol. 2, A. Hirschwald, Berlin 1876, pp. 525-540.
- ↑ Paul Julius Möbius: About the hereditary nervous diseases. Breitkopf and Härtel, 1879.
- ↑ A. Ferbert, W. Kress: Clinic and genetics of limb-girdle muscular dystrophy. In: Medical Genetics. Volume 21, Number 3, 2009, p. 332, doi: 10.1007 / s11825-009-0171-x