Glycidyl trimethyl ammonium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
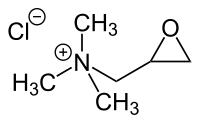
|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Glycidyl trimethyl ammonium chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 14 ClNO | |||||||||||||||
| Brief description |
odorless whitish solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 151.63 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.13 g cm −3 |
|||||||||||||||
| Melting point |
133-137 ° C |
|||||||||||||||
| solubility |
soluble in water (852 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Glycidyltrimethylammonium chloride is a chemical compound from the group of substituted quaternary ammonium salts .
Extraction and presentation
Glycidyltrimethylammonium chloride can be synthesized from epichlorohydrin , trimethylamine, and water. Hydrochloric acid and sodium hydroxide are required as catalysts . Commercial products contain 3-chlorohydroxypropyltrimethylammonium chloride (<4%), 2,3-dihydroxypropyltrimethylammonium chloride (<3.5%) and some other compounds (e.g. traces of epichlorohydrin) as impurities.
properties
Glycidyltrimethylammonium chloride is a flammable, odorless, whitish, hygroscopic solid. It decomposes after melting.
use
Glycidyltrimethylammonium chloride is mainly used for the cationization of starches in the paper industry. In 2001 about 6000 tons of the compound were used for this. It is usually sold as a 75% solution in water.
safety instructions
There are some known cases of dermatitis and sensitization caused by the commercial handling of glycidyltrimethylammonium chloride despite working under safety precautions. The compound is directly mutagenic and carcinogenic.
Individual evidence
- ↑ a b c The MAK Collection for Occupational Health and Safety: Glycidyltrimethylammoniumchlorid (MAK Value Documentation in German language, 1991), doi : 10.1002 / 3527600418.mb303377d0017 (free full text).
- ↑ a b c d e f Entry on glycidyltrimethylammonium chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b c d e ECHA: EU RISK ASSESSMENT , accessed on August 16, 2014.
- ↑ Entry on 2,3-epoxypropyltrimethylammonium chloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .


